Introduction
Citrus fruits are the second most cultivated fruits which have a global large-scale market (Hyun et al., 2011). In South Korea, many mandarin have been produced from cultivation area of about 25,000 ha in Jeju island. The industry scale of the citrus market is about 1 trillion won, contributing most of agricultural income (Hyun et al., 2011; Kwon et al., 2003). Therefore, management of citrus cultivation to maintain citrus productivity and profitability is very important.
Of the various factors responsible for decreasing citrus production, disease is important cause not only damage to fruit and also decrease of productivity and income. Disease infections are a major challenge to citrus production worldwide. In South Korea, approximately 35 type of disease are known to damage citrus fruit (Nam et al., 2009). Among these, the major damaging disease, Diaporthe citri is generally known to cause melanose (Huang et al., 2013). The pathogen produces the small black lesions raised pustules on the leaves, twigs and fruits (Timmer et al., 1988). Especially, the biggest problem is the damage of fruit, and it occurs during heavy rainfall from June to August which is a fruit growing seaseon (Kwon et al., 2003). Therefore, in order to control this, chemical fungicides such as Mancozeb are treated on citrus tree 4-6 times in Jeju. However, increased use of chemical pesticides to control these disease causes several negative effects such as the development of pathogen resistance, deterioration of human health, and environmental risk (Gerhardson, 2002).
Therefore, Use of functional microorganisms is being considered as a suitable alternative for reducing the use of the chemical fungicides, contributing to an eco-friendly agriculture (de Weger et al., 1995). Various kinds of microorganism such as plant growth promoting rhizobacteria (PGPR) have many potential uses as agents for enhancing plant growth and managing plant disease (Glick, 1995; Kim et al., 2011; Weller, 1988). PGPR which used as biocontrol agent produced cell degrading enzymes include lytic activity (Woo et al., 2007) and antibiotics to restrict the growth of pathogens (Ko et al., 2009). Their mechanisms also include competition for space and nutrients (Jung et al., 2006) and inducing systemic resistance (ISR) (Ping and Boland, 2004).
Even though most studies have focused on the efficacy of biocontrol, field application has not been investigated. Microorganism can easily synthesize their metabolites (enzymes and antibiotics) under laboratory conditions but, they hardly produce sufficient metabolites under field conditions. Therefore, in the present study, we develop the large-scale fermenter, cost-effective growth medium based on fertilizer for field application and determined the ability of Bacillus amyloliqeufaciens Y1 incubated in cost-effective fermenter against citrus melanose caused by Diaporthe citri.
Materials and Methods
Cost-effective fermenter with large-scale and growth medium based on fertilizer
To reduce the cost of cultivation, a low-cost PB medium was developed using fertilizer for mass cultivation of bacterial strains. The composition of PB medium (per 500 L) was 1,500 g compound fertilizer 100 g MgSO4, 100 g KH2PO4, 50 g CaCO3, 50 g NaCl, 1,500 g sucrose, 300 g KOH, 12 g of gelatin, 250 g chitin powder, 300 g yeast extract and 1 L of Power Chitin (Purune, South Korea) (Table 1). The low-cost fermenter (1 ton) for large-scale (in field) cultivation was developed as a model name, M-1000 (MC-Biotec, South Korea). The experiment was conducted by using M-1000 fermenter containing 500 L non-sterilized PB medium.
Table 1. Composition of PB medium based on fertilizer.
| Fertilizer | Quantity |
| Composite fertilizer (20-20-20) | 1 kg |
| MgSO4 | 100 g |
| CaCO3 | 50 g |
| NaCl | 50 g |
| Sucrose | 1 kg |
| Chitin Powder | 250 g |
| Gelatin | 12g |
| Power Chitin (Prune) | 1 L |
Investigation on growth and pH
To investigate the pattern of the cell growth and changes in pH according to the incubation days in small-scale condition (in lab), the laboratory TSB medium and low-cost PB medium were used for inoculation of Y1. Each medium was sterilized by autoclave. The identical colonies of Y1 (2 mm) were used for lab-scale inoculation. Then, 500 mL of TSB and PB medium was prepared and 5 colonies inoculated.
In large-scale inoculation of Y1, 100 µL (107 CFUs mL-1) of strain Y1 was spread on TSA plates (150 mm diameter) incubated for 3 days at 30°C. Two bacterial plates were inoculated in large-scale fermenters with 500 L of non-sterilized PB medium. Each conditions were incubated at 50°C and adjusted to initial pH 7.5 with KOH. The samples were taken from each culture broth daily for 10 days and observed cell numbers and changes of pH. The experiment was performed in triplicate.
Mycelial growth inhibition by culture filtrate on D. citri
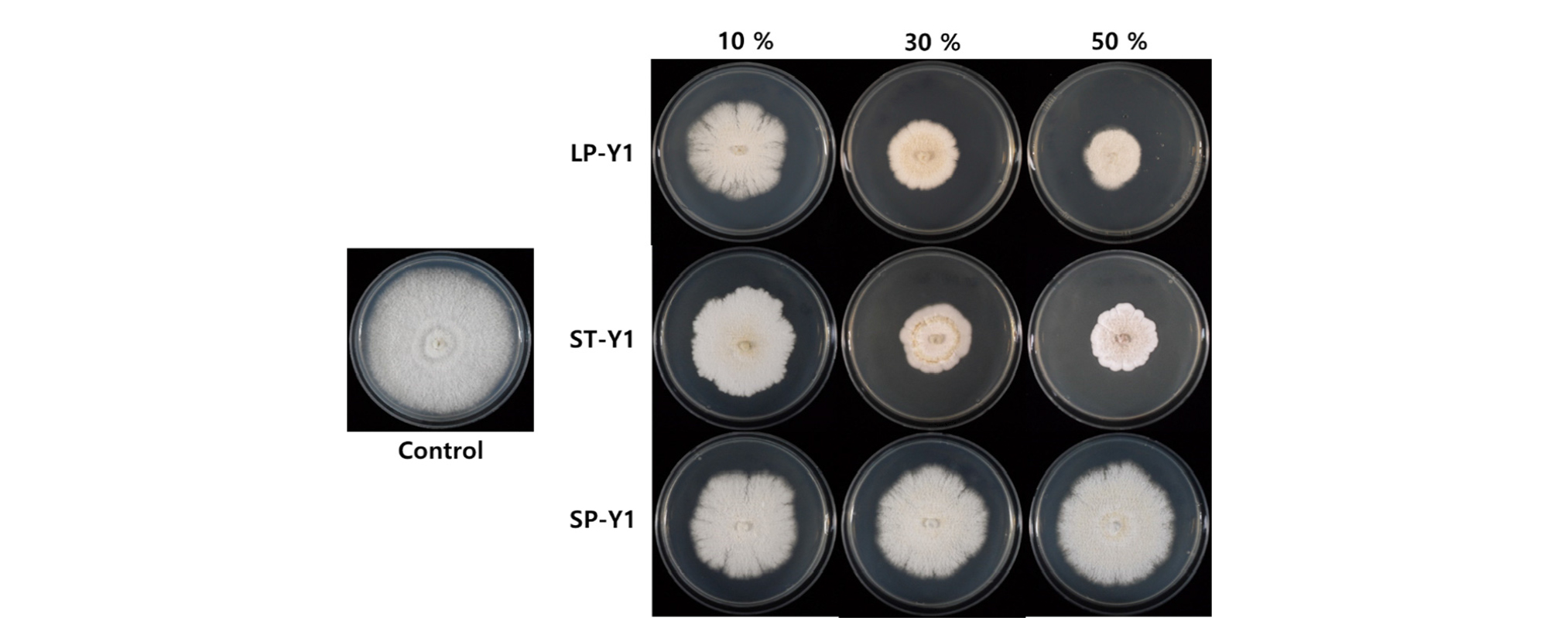
To investigate the inhibition of mycelial growth of the fungal pathogens, the supernatant which bacterial culture grown for 7 days on small scale and large scale were collected using centrifuge at 12,000 rpm for 15 minutes and the supernatant was filtered with Whatman paper No. 6. A syringe filter was used to completely remove remaining cells. The final culture filtrate was mixed with pre-autoclaved PDA and cooled to 60°C to adjust the final concentrations to 10%, 30% and 50%. Then, D. citri fungal plug (2 mm) was inoculated in the center of each plate. Only pathogen on PDA plate without bacterial culture filtrate (0%) was used as control. Mycelial growth inhibition was measured at 14 days after inoculation and inhibition percent was determined based on the following equation, Inhibition percent (%) = (C-T) / C × 100 (C = pathogen diameter in control plate, T = pathogen diameter in treatment plate)
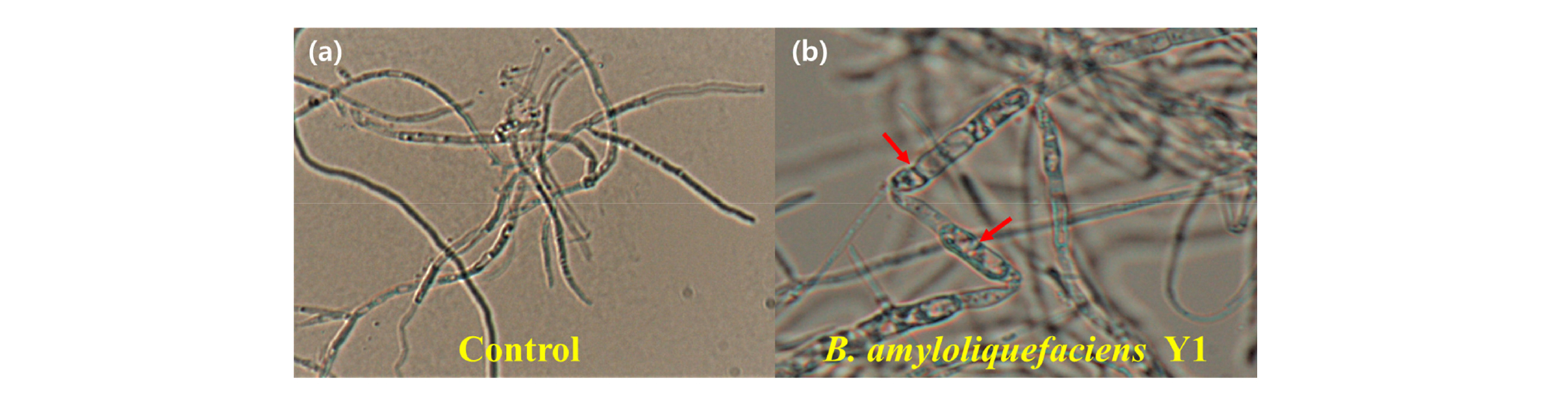
Then, the hyphal deformation of the mycelium from each plate was examined under the microscope.
In vivo experiment
Three years old mandarin seedlings were taken from Jeju Island, South Korea and transplanted in 30 cm × 25 cm plastic pots. The soil composition was 2:1:1 ratio of bed soil, vermiculite and granite fragments. The mandarin pots were grown under glasshouse condition in Chonnam National University for 1 year and the plants were watered one-week interval. To induce conidial production of Diaporthe citri was followed method of Ko et al. (2012).
The identical 4 years-old mandarin plants in the pots were used to investigate for melanose disease control by the culture broths of strain Y1. When the plants have young leaves, treatments were applied to evaluate disease control. Three types of treatment as Y1 culture broth (Y1), synthetic fungicide (F) and control (Con) were used in this experiment. The strain Y1 was grown in large-scale fermenter using PB medium for 7 days at 50°C. Bacterial culture broth (2.0 × 105 CFUs·mL-1) which was mixed with distilled water (1:1 v:v) was treated on the surface of the leaves and dried for 2 h. The synthetic fungicide, Dithianon was prepared according to the label recommendation and sprayed on the leaves and dried for 2 h. The plants sprayed with water were used as control. Then, the conidial suspension of D. citri (1.0 × 105 conidia·10-1) was sprayed on the surface of the leaves in each plant of every treatment. The inoculated plants were maintained at 28 ± 2ºC and 60% relative humidity. The disease incidence was determined by counting the number of leaves having melanose symptoms on the plants of each treatment.
Statistical analysis
Experimental data were analyzed by standard analysis of variance (ANOVA) followed by the least significant difference (LSD) tests at p < 0.05 using the statistical analytical system (SAS. Version 9.4).
Results and Discussion
Growth pattern and pH changes by small-scale and large-scale cultivation
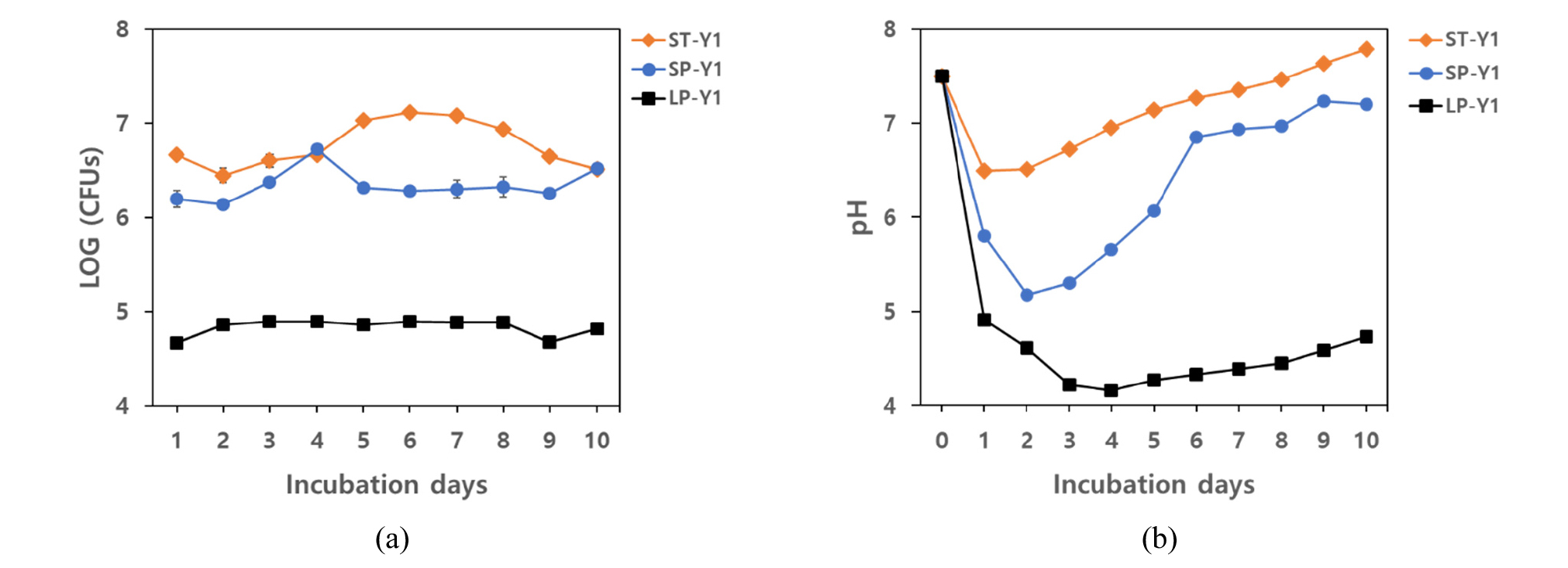
In the small-scale experiment (In lab), the growth of Y1 in TSB medium rose steadily from 2 DAI till 7 DAI and reached a peak (1.3 × 107 CFUs/mL) at 6 DAI. Nonetheless, the growth pattern of Y1 in PB medium was almost persistent till the end of the experimental period with the exception at 4 DAI of which was the highest rate (5.33 × 106 CFUs/mL) in PB medium. The value of pH sharply declined at 1 DAI in both TSB and PB medium, but the value was continuously decreased and reached the lowest value (5.17) at 2 DAI in PB medium. The pH value increased slowly from 3 DAI in both media and reached almost the value of the initial pH at the final day of inoculation. In 500 L large-scale cultivation (In Field), the strain Y1 was suddenly increased in cell numbers (7.3 × 104 CFUs/mL) at 2 DAI. From 2 DAI to the final day of the experiment, the growth was stable with a slight decrease at 5 DAI and 10 DAI and the range of the cell numbers was between 7.3 × 104 CFUs/mL and 7.9 × 104 CFUs/mL (Fig. 2). The pH of the medium inoculated with the strain Y1 instantly decreased at 1 DAI and the continuous decline occurred till 4 DAI at which the pH was the minimum value (4.16). At 5 DAI, the pH slowly increased again till the end of the experimental period (Fig. 2). The all treatment was incubated without contaminations (Fig. 3).
Although various types of microbial products have been introduced to the market, there are many problems related to high cost of products and fermenters, contamination during incubation, loss of consistency and low efficiency in the field (Jeon, 2017; Ryu and Gyehun, 2000). The other limitation to mass cultivation of biocontrol agents is inefficient cost of the fermenter and growing medium. Suitable medium for large-scale production of biocontrol strain should not only be inexpensive but also efficient for production of metabolites (Lewis, 1991). The results of this study showed mass cultivation of Y1 using non-sterile medium without contamination to produce sufficient cell density for field application. Therefore we demonstrate solutions for minimizing contamination and cost problems during the mass cultivation of biocontrol agents for field application.
Mycelial growth inhibition
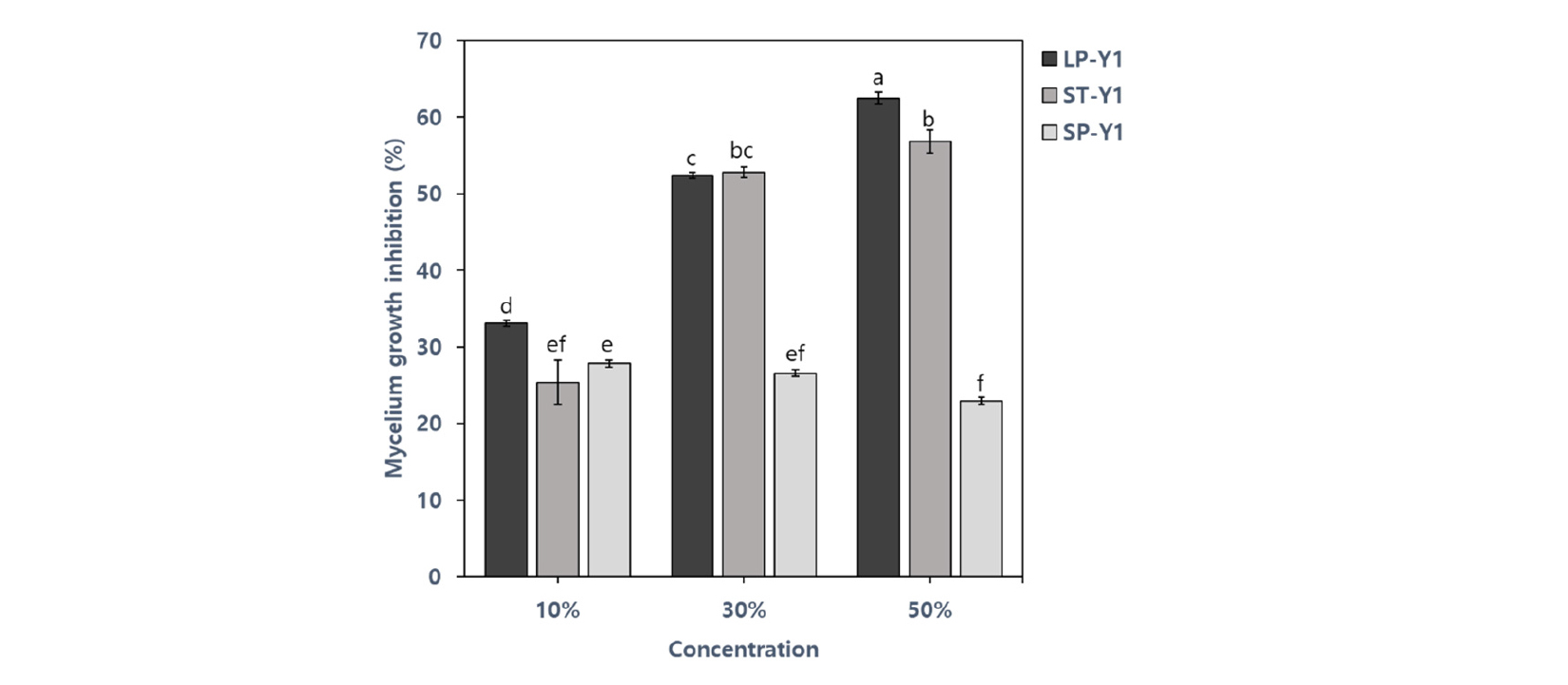
Previous reports have shown that B. amyloliquefaciens Y1 inhibits mycelial growth of several fungal pathogens such as Rhizoctonia solani, Colletotrichum gloeosporioides, Botrytis cinerea, Phytophthora capsici, Fusarium oxysporum and Fusarium graminearum (Jeon, 2017). The BCF of B. amyloliquefaciens Y1 showed the effect in reducing the mycelial growth of D. citri. The large-scale cultivation of the strain Y1 in PB medium (LP-Y1) showed higher inhibition than those of small-scale cultivation in PB medium (SP-Y1) which had the lowest inhibition rate. In case of the cultivation period, Mycelial growth inhibition using 50% BCF from LP-Y1 (62.5%) was the strongest to restrict the mycelial growth of D. citri. Inhibition effect from lab-scale cultivation in TSB medium (ST-Y1) was also effective to control mycelial growth (56.85 %) (Fig. 4). As shown in figure 6, mycelial structure was dilated and transformed due to BCF of Y1 strain compared to the thin and straight control of mycelium under a microscope. Similarly, Nam et al. (2009) also demonstrated that the strain Bacillus subtilis KB-401 strongly inhibited mycelial growth of D. citri.
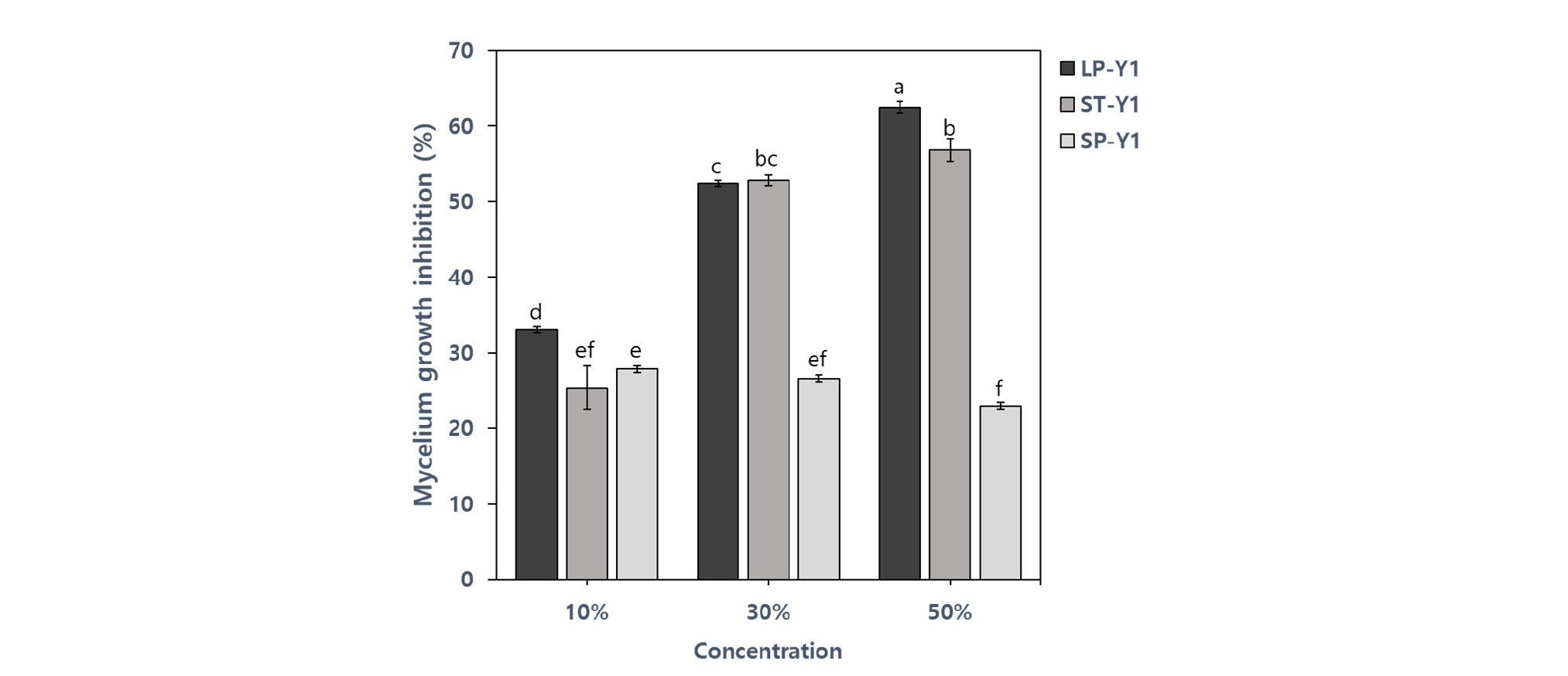
Fig. 4.
Mycelial growth inhibition of D. citri by different bacterial culture filtrate concentrations of the strain Y1 from the large-scale cultivation in PB medium (LP-Y1), the small-scale cultivation in TSB medium (ST-Y1) and PB medium (SP-Y1) of after inoculation 7 days. The values represent the means calculated from three replicates and means with the same letter are not significantly different at p ≤ 0.05 by least significant difference test.
As a result, the ST-Y1 cell number was higher than LP-Y1, but the antifungal activity was highest in LP-Y1. This can be attributed to the production of more metabolites related to pathogen inhibition in the field. Bacillus amyloliquefaciens Y1 is known to produce antibiotics and releases a variety of antimicrobial compounds such as iturins, cyclo (D-Pro-L-Val) and cyclo (D-Pro-L-Leu) (Jamal et al., 2017a; Jamal et al., 2017b; Jeon, 2017). By optimizing the required environmental conditions related bacterial incubation, large quantities of the desired metabolites can be easily produced in vitro. However, it is not practical to develop these specific conditions in the field. As a result, the number of inoculated biological control strains is significantly less than that of indigenous microorganisms in the soil, reducing their effectiveness. An increase in population of biocontrol strain leads to the enhanced pathogen inhibition (Ahmad et al., 2011). In this study, we not only demonstrated the large-scale culture of Y1 with no contamination, but also confirmed the effect of sufficient cell densities and adequate metabolites for field application.
In vivo biocontrol efficiency against D. citri
As shown Fig. 7, the treated with B. amyloliquefaciens Y1 on mandarin plants had a strong inhibitory effect on melanose disease causing D. citri. The lowest incidence was 3.51% in plants treated with fungicide. Treatment with the biological control strain Y1 (11.25%) significantly reduced the disease incidence rate of the plants compared to the control treatment with the highest incidence (46.28%) at 20 days after the inoculation of the pathogen. There is no significant difference between treatments using biocontrol strain and fungicide, so Y1 strain can be used as a fungicide replacement agent. Other report by Ko et al. (2014) also showed that treatment with Pseudomonas putida THJ609-3 effectively reduced the incidence of melanose in citrus leaves and suppressed spore germination of D. citri. The results of our pot experiment proved that pre-treatment with Y1 culture broth by foliar application significantly reduced the incidence of melanose in citrus plants. From our results, culture broth and various fungal metabolites from Y1 may have played an important role in regulating D. citri.
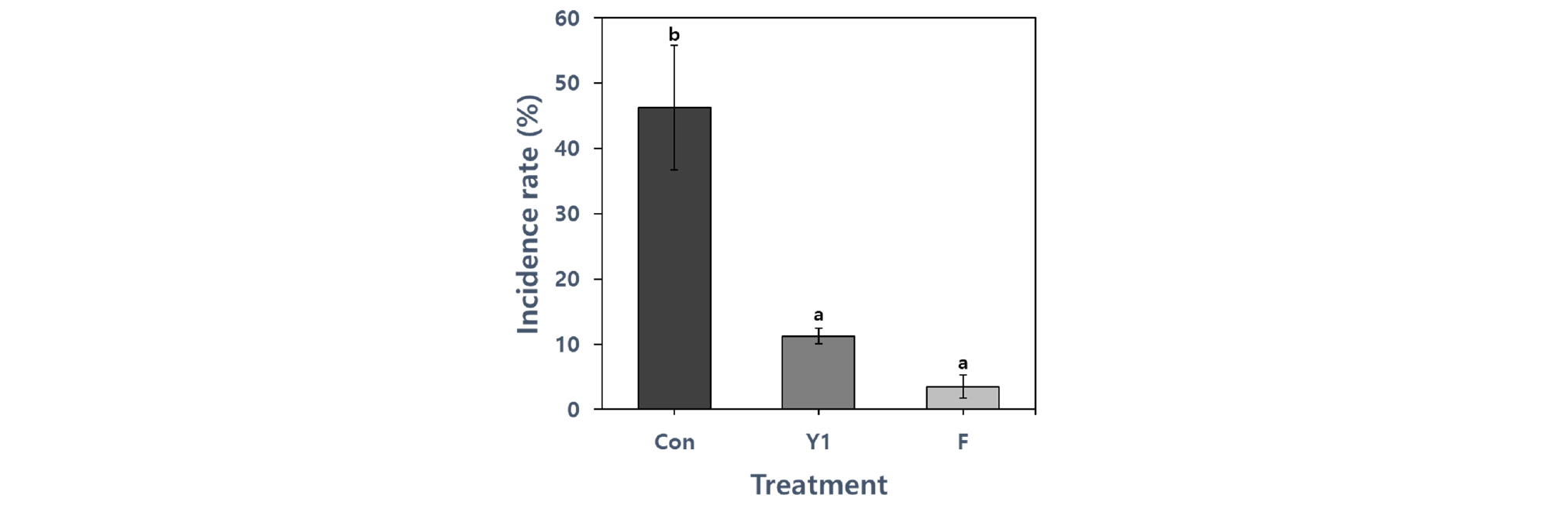
Fig. 7.
Effect of different treatments on Diaporthe citri disease incidence rate of mandarin plants. Statistical comparison between treatments was done by least significant difference (LSD) test at p < .05 level. Means with the same letter are not significantly different from each other. * Control (Con), Y1 treatment (Y1), and Fungicide treatment (F).
Conclusion
In this study, large-scale mass cultivation of strain Y1 and medium based on fertilizer which can be applied to the field at low cost were developed to control citrus melanose disease. The results of this study demonstrate that B. amyloliquefaciens Y1 can be successfully grown on a large-scale using fertilizer medium without contamination, producing sufficient metabolites for inhibition of D. citri under in vitro and in vivo conditions. Our results also showed optimized conditions for cost-effective large-scale cultivation of Y1 as compared to cultivation using commercial medium, and successfully controlled citrus melanose disease. Further research is required to identify the antifungal compounds from mass culture, clarify the mechanism of inhibition and verify the consistent performance of these cultures in the field.