Introduction
Materials and Methods
Experimental setup
Physicochemical properties of soil
Macro nutrients, soluble sugars and proline of barley and oat
Statistics
Results and Discussion
Soil chemistry
Physiological response of barley and oat plants in the reclaimed soil
Nitrogen use efficiency and PCA analysis
Conclusions
Introduction
Salinization of agricultural soils is a global concern that leads to poor crop growth and production, and its occurrence is due to natural geochemical processes including elevated sea level and saltwater intrusion and antropogenic activities such as excessive fertilizer, poorly land management and intensified agricultural practices (Machado and Serralheiro, 2017; Hopmans et al., 2021; Singh, 2022). Generally, an accumulation of water-soluble salts including Na+, K+, Cl- and SO42- in the rhizosphere causes the disruption of water (osmotic) potential that strongly restricts water flow from soil to plant roots (Munns and Tester, 2008; Stavi et al., 2021).
The reclaimed agricultural lands in South Korea accounts for approximately 7.1% (112,464 ha) (MOLIT, 2021), and the Saemangeum reclaimed land (8,570 ha) is partly used for research or under development (Lie et al., 2008). Due to high salinity, the reclaimed soil should firstly be amended to reduce limiting factors for crop production (Koo et al., 1998) as well as to improve bad drainage (Kotb et al., 2000).
Plants have developed various strategies to alleviate salt stress, and, nevertheless, most crop species still experience undesirable consequences of salt stress; morphology (poor growth and chlorosis), physiology (reduced photosynthesis and nutrient imbalance) and biochemistry (oxidative stress and membrane disintegrity) (Hannachi et al., 2022; Ji et al., 2022). Of defense mechanisms, the accumulation of compatible organic solutes such as soluble sugars, proline and glycine-betaine is most ubiquitous response in plants grown under higher salt environments. These soluble compounds not only play as osmo-protectants but also contribute to the intracellular osmotic adjustment and ROS detoxification (Abdelhamid et al., 2013; Sharma et al., 2019; Singh et al., 2022).
Among essential elements to require plant growth and development, nitrogen (N) is well known as most important nutrient to involve a variety of physiological metabolism, and thus, the difference in N availability from soil and/or fertilizer usually limits crop yield in most agricultural production systems (Robertson and Vitousek, 2009). Application of N fertilizer become an important practical measure to ensure potential crop yield, however, excessive N dose resulted in serious environmental concerns rather than yield improvement due to lower nitrogen use efficiency (NUE) (Vitousek et al., 2009; Good and Beatty, 2011). Therefore, new approaches should be developed to achieve improved crop productivity and NUE with detailed assessment.
Barley (Hordeum vulgare L.) is the fourth most important cereal crop in the world. However, the cultivated area, production and self-sufficiency rate in South Korea are estimated as 23,639 ha, 67,984 Mg and 38.2%, and the cultivated area of oat (Avena sativa L.) is only 1,840 ha (KOSIS, 2021). Barley is known as cold-resistant crops and represent a predominant viability under poor conditions such as arid and saline soils, this trait of barley can mitigate the harmful effect of salinity by excluding from absorption or accumulating in tissues (Chen et al., 2007). By contrast, the growth including germination and development of oat were directly and greatly inhibited by drought and saline-alkali conditions (Zhao et al., 2013; Zhang et al., 2018).
Both barley and oat are increasingly expanding crops due to the advantage as healthy food crops such as lowering blood sugar and lipids and regulating blood pressure as well as raising self-sufficiency rate. However, many difficulties still remain to ensure favorable production of both cereal crops in newly reclaimed land like Saemangeum. Therefore, this study is aimed to investigate physiological responses which are emphasized on osmo-protectants and NUE from selected barley and oat varieties, and to compare between varieties by principal component analysis (PCA) to find what variety is suitable for a cultivation in the reclaimed land.
Materials and Methods
Experimental setup
This work was carried out at the reclaimed experiment field of National Institute of Crop Sciences (NICS), Gimje, Jeonbuk province from late March to late June, 2023. As an experimental crop, barley (Hordeum vulgare cv. Jaeanchal, Sogang) and oat (Avena sativa cv. Daeyang, Joyang) were selected and seeds (130 kg ha-1) were sown in rows (strip sowing). Each plot was designed with 40 m2 (4 m of width × 10 m of length) with a randomized complete design (three replications). Fertilization was implemented with the standard application rates (N-P-K = 88-72-36 kg ha-1) based on NAS (2022) recommendation.
Physicochemical properties of soil
Composite and core soil samples were collected from three depths (0 - 20, 20 - 40, 40 - 60 cm). Soil texture was determined with hydrometer method. Soil bulk density (BD) was measured using the soil core method (Grossman and Reinsch, 2002). Soil chemical properties were analyzed with a recommended preparation according to NAS manual (Lee et al., 2017a). The pH and EC were measured with pH/EC meter after 30 min of shaking (sampled soil:ddH2O = 1:5). The inorganic N was measured with automatic-nitrogen analyzer (440 nm, Auto analyzer 3, BRAN+ LUEBBE, Germany) after 30 min of shaking (5 g of sampled soil + 25 mL of 2 M KCl) and filtering (Whatman No. 2). The soil total carbon (dried soil) was measured C/N analyzer (vario Max CN Element Analyzer, Elementar GmbH, Germary), and transformed to organic matter with multiplying by 1.724. For available phosphate, 5.0 g of dried soil was mixed with 20 mL of Lancaster solution (330 mM acetic acid + 1.5 N lactic acid + 30 mM of ammonium fluoride + 213 mM sodium hydroxide + 50 mM ammonium sulfate, pH 4.25), shaken for 10 min, filtered (Whatman No. 2) and measured with UV-Spectrometer (720 nm, UV 1900i, Shimadzu, Japan). The cations (5 g of dried soil) were analyzed with ICP (GBC, Intergra XL. Australia) after a series of extraction (25 mL of 1 N CH2COONH4, pH 7.0), shaking (30 min) and filtering (Whatman No. 2).
Macro nutrients, soluble sugars and proline of barley and oat
For analyzing contents of mineral nutrients in shoot, root and spike, dried plant samples (0.5 g) were firstly mixed with 5 mL of a extraction solution (377 mM H2SO4 + 36% perchloric acid), wet digested with stepwise increasing of temperature (150 - 250°C), filtered (Whatman No. 6) after cooling at ambient temperature, and adjusted to 100 mL with ddH2O (Lee et al., 2017b). Nitrogen was measured with automatic-nitrogen analyzer (440 nm, Auto analyzer 3, BRAN+ LUEBBE, Germany), phosphorus was measured with UV-Spectrometer (720 nm, UV 1900i, Shimadzu, Japan), and cations were measured with ICP (GBC, Intergra XL. Australia). In order to determine carbohydrate contents, the dried leaf samples (0.2 g) were first boiled with 10 mL of 80% EtOH in boiling water. The alcoholic extracts were evaporated under boiling water bath, and the residues were re-dissolved with distilled water. Water extracts were mixed with 2 volumes of 0.2% anthrone in a concentrated H2SO4 followed by estimation of carbohydrate as described by Roe (1955). Glucose was used as standard for both soluble sugars and starch. The proline was analyzed with a previous method (Carillo and Gibon, 2011). The fresh samples (0.5 mg) from rice shoots and roots were mixed with 1 mL of 40% ethanol (v/v) at 4°C for 24 h, centrifuged at 14,000 × g for 5 min, and the supernatant was extracted. The supernatant (500 µL) was immediately incorporated into a reaction solution containing 3% of sulfosalicylic acid, 1 mL of glacial acetic acid and 1 mL of acidic ninhydrin, incubated at 96°C for 1 h, and the reaction was terminated on ice. Toluene (1 mL) was added to the reaction mixture, and the absorbance of the supernatant was measured at 520 nm using a spectrophotometer (UV-1900i, Shimadzu, Japan) after vortexing for 20 s and standing for 5 min. The L-proline was used as a standard (Sigma, MO, USA).
Statistics
The experimental design was a completely randomized design with three replications. Data were subjected to a T-test (RStudio, v.4.0.4), and the contribution of variables was determined by principal components analysis (RStudio, v.4.0.4). Graphs were generated using Sigma Plot (v. 14.5, SYSTAT, Palo Alto, CA, USA).
Results and Discussion
Soil chemistry
Indeed, physical properties were poor for crop production due to soil texture (silt loam) and low water and nutrient holding capacity. Chemical properties were also not suitable due to significantly low level of EC, TC and TN, including inorganic N, compared to NAS recommendation (NAS, 2022) (Table 1). Compared to before experiment, pH, available P2O5 and exchangeable K+ showed an increased tendency at harvest stage. Soil chemistry by soil depth also did not show any marked changes. Relative greater total carbon (TC) in top soil at harvest stage was believed that crop residue (root) was mixed in soil sample. The poor development of soil layers and aggregation of newly reclaimed lands not only decreases water and air permeability of soil but also raises groundwater level (Son et al., 2005), and an incorporation of organic matter like crop residue was suggested as a countermeasure for soil aggregation (Plante and McGill, 2002; Ashman et al., 2003; Son and Cho, 2009). Taken previous reports together, it could be implied that improving soil physical properties in the reclaimed land should be prioritized.
Table 1.
Soil physico-chemical properties at before and after experiment.
|
Soil depth (cm) |
Soil tex- ture |
Bulk density (g cm-3) |
Poro- sity (%) |
pH (1:5) |
EC (dS m-1, 1:5) |
TC (%) |
TN (%) |
NO3--N (mg kg-1) |
NH4+-N (mg kg-1) |
Avail. P2O5 (mg kg-1) | Exch. cations (cmolc kg-1) | ||||
| K | Ca | Mg | Na | ||||||||||||
| Before | 0-20 |
Silt loam | 1.23 | 54 | 6.9±0.0 | 0.3±0.0 | 0.5±0.1 | 0.06±0.00 | - | 8.3±0.2 | 190±24 | 0.5±0.0 | 3.3±0.0 | 2.05±0.01 | 0.1±0.0 |
| After | 0-20 | 1.23 | 54 | 7.2±0.0 | 0.2±0.0 | 0.8±0.2 | 0.05±0.02 | 0.8±1.7 | 3.8±0.8 | 382±110 | 0.6±0.0 | 4.3±0.1 | 2.01±0.08 | ND† | |
| 20-40 | 1.31 | 50 | 7.4±0.1 | 0.2±0.0 | 0.6±0.2 | 0.04±0.01 | 0.9±1.6 | 3.6±0.7 | 319±116 | 0.6±0.0 | 4.3±0.1 | 2.13±0.05 | ND | ||
| 40-60 | 1.31 | 50 | 7.5±0.1 | 0.2±0.1 | 0.5±0.2 | 0.03±0.01 | 0.4±0.5 | 4.1±0.8 | 226±71 | 0.6±0.0 | 3.8±0.0 | 2.22±0.04 | 0.1±0.0 | ||
| Optimal | - | - | - | 6.5-7.0 | - | 0.2-0.3 | - | - | - | 150-250 | 0.45-0.55 | 6.0-7.0 | 2.0-2.5 | - | |
Physiological response of barley and oat plants in the reclaimed soil
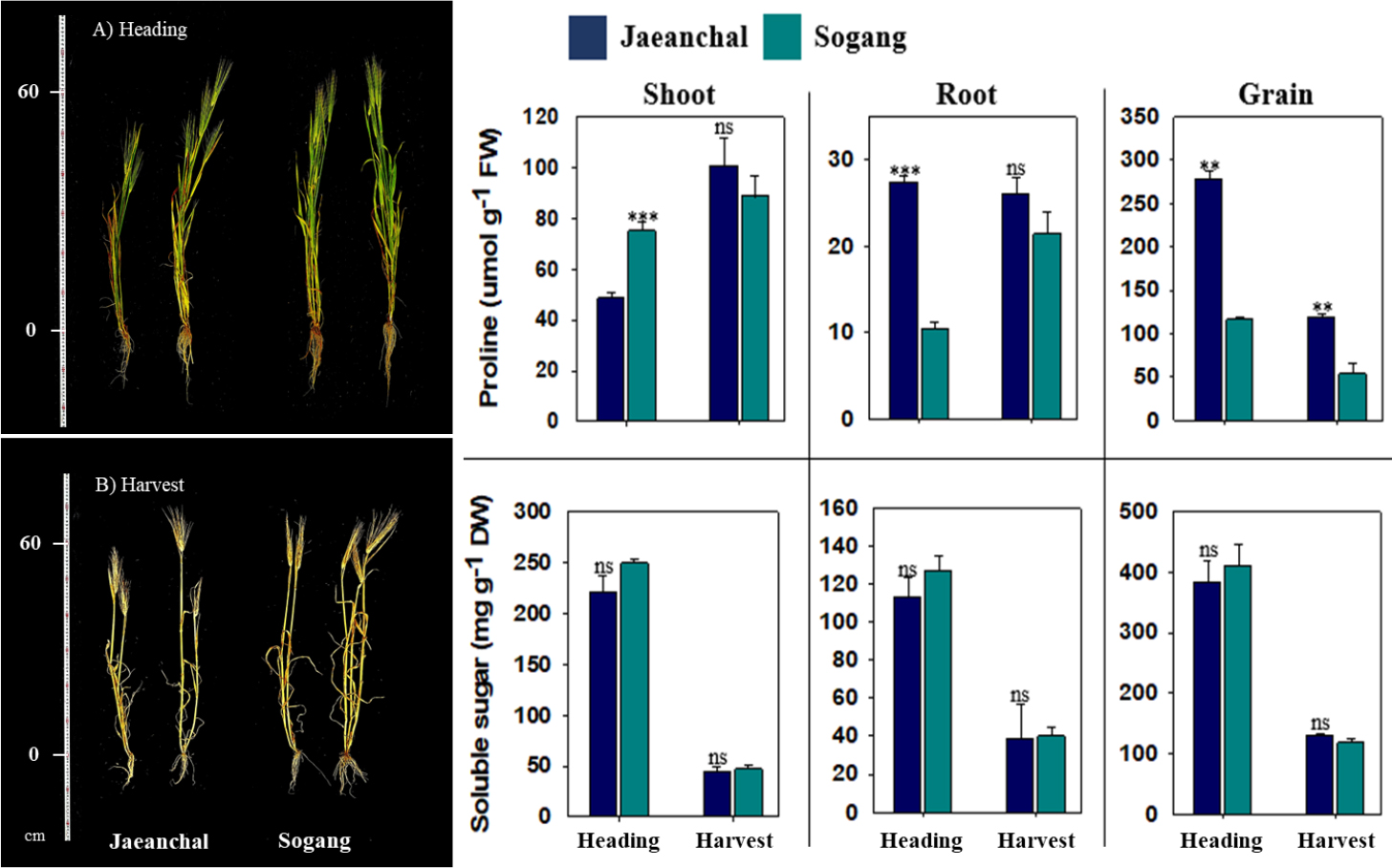
Growth (shoots and roots) and biomass production at heading stage were not differed from barley varieties (Table 2). By contrast, at harvest stage, spike yield was significant greater (p < 0.05) in Sogang (4,200 ± 173 kg ha-1) compared to Jaeanchal (2,920 ± 481 kg ha-1) although the other growth indices were not different. Macro nutrients were measured from shoot, root and spike of both barley varieties at harvest stage (Table 3). Significant difference in tissues and each element was not observed between both varieties. Overall, the concentrations in nitrogen (N) and phosphate (P2O5) showed a trend in the highest in grain (1.92 - 2.31% and 1.07 - 1.08%, respectively), whereas potassium (K2O, > 3.5-fold greater) and sodium (Na2O, >2.0-fold greater) were markedly concentrated in shoot. Additionally, calcium (CaO) was dominantly allocated in vegetative tissue (shoot and root, 5 times greater), and magnesium (MgO) showed an equal distribution among tissues. Proline was remarkably perturbed by varieties, tissues and growth stage (Fig. 1). The highest proline level was observed in spike followed by shoot and root. In spike, Jaeanchal showed significantly higher abundance compared to Sogang at both heading (2.5-fold) and harvest (2.0-fold), whereas the level was differed from varieties at heading stage (shoot in Sogang and root in Jaeanchal), whereas soluble sugars in all parts represented the similar trend between varieties, and only greatly differed from growth stage (heading > harvest). By contrast, barley, growth and biomass production varied between oat varieties at heading stage (p < 0.05) although they were not at harvest stage (Table 4). Daeyang showed greater production of vegetative tissues, whereas Joyang produced more spikes. Even though there was not statistic difference at harvest stage, Joyang showed a tendency of greater ear production. The concentrations in macro nutrients showed a significant difference in some nutrients of shoot and root at harvest stage (Table 5). Total N was 2.5-fold higher concentration in the shoot of Daeyang (1.01 ± 0.21%) compared to Joyang (0.39 ± 0.08%), in contrast, Joyang showed significant abundance in K2O (5.29 ± 0.65%) and CaO (0.39 ± 0.03%) compared to Daeyang (3.79 ± 0.19% for K2O and 0.27 ± 0.01% for CaO). By contrast, K2O concentration in root was markedly higher in Daeyang (2.27 ± 0.27%) compared to Joyang (1.11 ± 0.21%). Proline in oat plant was obviously abundant in Daeyang in all tissues, and its level was significant accumulated at harvest stage (Fig. 2). Similar to barley, an abundance was the highest in spike followed by shoot and root. In oat varieties, the level of soluble sugars at heading stage differed depending on the tissues, with higher in root (Joyang) and higher in spike (Daeyang). On the other hand, soluble sugars in Daeyang at harvest stage were significantly higher accumulated in all tissues. The reduction in grain yield of barley was diverse from 28 to 80% depending on variety sensitivity and saline concentration (Tavakoli et al., 2010; Hammami et al., 2017; Vasilakoglou et al., 2021). Sohn et al. (2009) documented that grain yield of barley and oat cultivated in the reclaimed land ‘Yeongsangang’ was equal to traditional agricultural lands, indicating a similar trend although our result showed spike yield including grain. It means that both cereal crops could be a suitable candidate for ensuring crop productivity in the reclaimed lands. Total N was significantly lower compared to a previous study (Yang et al., 2012), and, moreover, the contents was greatly dependent upon soil properties (reclaimed land-specific) (Shin et al., 2005). In addition, lower Na2O content compared to previous study (Yang et al., 2012) is not likely to limit not only crop growth but also ion balance. In general, proline accumulation is one of biochemical mechanisms coping with reactive oxygen species (ROS), and salt-tolerant plants accumulate compatible solutes including proline, consuming less energy under salt stress. Previous studies have found that, under stress conditions, proline accumulation contributes to stabilizing cellular structures and scavenging free radicals (Ashraf and Foolad, 2007; Binott et al., 2017; Shena et al., 2018). In contrast with these reports, higher biomass-produced barley and oat variety accumulated lower proline, and this implied that an experiment land did not drive a negative effect on limited growth and production, suggesting a possibility for the cultivation of both crops.
Table 2.
Growth and biomass production of barley (Hordeum vulgare) varieties grown under the reclaimed soil.
| Stage | Variety | Shoot length (cm) | Root length (cm) | Dry weight (kg ha-1) | ||
| Shoot | Root | Spike | ||||
| Heading | Jaeanchal | 68.8 ± 9.0 | 24.8 ± 3.6 | 5,584 ± 531 | 549 ± 217 | 2,358 ± 537 |
| Sogang | 68.6 ± 7.5 | 24.6 ± 0.2 | 5,476 ± 285 | 503 ± 78 | 2,311 ± 472 | |
| t-test | ns† | ns | ns | ns | ns | |
| Harvest | Jaeanchal | 64.5 ± 6.0 | 14.1 ± 2.9 | 3,350 ± 696 | 223 ± 63 | 2,920 ± 481 |
| Sogang | 65.2 ± 6.8 | 14.2 ± 0.3 | 4,034 ± 100 | 402 ± 180 | 4,200 ± 173 | |
| t-test | ns | ns | ns | ns | * | |
Table 3.
Tissue-specific macro-elements (%, DW) of barley (Hordeum vulgare) varieties grown under the reclaimed soil at the harvest stage.
| Tissue | Variety | T-C | T-N | P2O5 | K2O | CaO | MgO | Na2O |
| Shoot | Jaeanchal | 39.6 ± 1.0 | 0.73 ± 0.24 | 0.62 ± 0.14 | 5.91 ± 0.81 | 0.64 ± 0.10 | 0.31 ± 0.05 | 0.12 ± 0.03 |
| Sogang | 41.3 ± 1.6 | 0.70 ± 0.12 | 0.51 ± 0.07 | 4.35 ± 0.63 | 0.60 ± 0.06 | 0.27 ± 0.03 | 0.12 ± 0.01 | |
| t-test | ns† | ns | ns | ns | ns | ns | ns | |
| Root | Jaeanchal | 40.4 ± 0.1 | 1.21 ± 0.22 | 0.37 ± 0.06 | 1.12 ± 0.24 | 0.69 ± 0.09 | 0.33 ± 0.04 | 0.06 ± 0.00 |
| Sogang | 41.9 ± 1.26 | 0.94 ± 0.15 | 0.35 ± 0.00 | 1.34 ± 0.17 | 0.64 ± 0.10 | 0.26 ± 0.06 | 0.07 ± 0.01 | |
| t-test | ns | ns | ns | ns | ns | ns | ns | |
| Ear | Jaeanchal | 41.7 ± 0.5 | 2.31 ± 0.15 | 1.07 ± 0.11 | 1.10 ± 0.08 | 0.13 ± 0.01 | 0.31 ± 0.02 | 0.03 ± 0.00 |
| Sogang | 41.5 ± 0.6 | 1.92 ± 0.41 | 1.08 ± 0.05 | 1.47 ± 0.37 | 0.13 ± 0.02 | 0.28 ± 0.03 | 0.04 ± 0.01 | |
| t-test | ns | ns | ns | ns | ns | ns | ns |
Table 4.
Growth and biomass production of oat (Avena sativa) varieties grown under the reclaimed soil.
| Stage | Variety |
Shoot length (cm) |
Root length (cm) | Dry weight (kg ha-1) | ||
| Shoot | Root | Spike | ||||
| Heading | Daeyang | 87.6 ± 0.8 | 22.1 ± 1.1 | 4,896 ± 82 | 560 ± 124 | 687 ± 160 |
| Joyang | 93.4 ± 1.5 | 20.2 ± 3.0 | 3,544 ± 423 | 246 ± 12 | 1,287 ± 61 | |
| t-test | *† | ns | * | * | * | |
| Harvest | Daeyang | 100.3 ± 3.6 | 13.8 ± 5.1 | 7,656 ± 1,212 | 363 ± 63 | 3,976 ± 405 |
| Joyang | 95.9 ± 7.9 | 17.5 ± 6.9 | 5,577 ± 880 | 381 ± 95 | 5,622 ± 930 | |
| t-test | ns | ns | ns | ns | ns | |
Table 5.
Tissue-specific macro-elements (%, DW) of oat (Avena sativa) varieties grown under the reclaimed soil at the harvest stage.
| Tissue | Variety | T-C | T-N | P2O5 | K2O | CaO | MgO | Na2O |
| Shoot | Daeyang | 41.2 ± 0.4 | 1.01 ± 0.21 | 0.86 ± 0.34 | 3.79 ± 0.19 | 0.27 ± 0.01 | 0.23 ± 0.04 | 0.03 ± 0.01 |
| Joyang | 40.6 ± 0.6 | 0.39 ± 0.08 | 0.54 ± 0.09 | 5.29 ± 0.65 | 0.39 ± 0.03 | 0.27 ± 0.02 | 0.11 ± 0.04 | |
| t-test | ns† | * | ns | * | ** | ns | ns | |
| Root | Daeyang | 38.6 ± 0.5 | 0.58 ± 0.10 | 0.59 ± 0.17 | 2.27 ± 0.27 | 0.55 ± 0.07 | 0.25 ± 0.03 | 0.06 ± 0.02 |
| Joyang | 38.2 ± 1.6 | 0.46 ± 0.06 | 0.42 ± 0.11 | 1.11 ± 0.21 | 0.69 ± 0.15 | 0.27 ± 0.03 | 0.05 ± 0.01 | |
| t-test | ns | ns | ns | ** | ns | ns | ns | |
| Ear | Daeyang | 44.1 ± 0.2 | 2.16 ± 0.09 | 0.77 ± 0.06 | 1.41 ± 0.13 | 0.24 ± 0.03 | 0.35 ± 0.04 | 0.02 ± 0.00 |
| Joyang | 43.8 ± 0.2 | 2.02 ± 0.28 | 0.86 ± 0.11 | 1.14 ± 0.12 | 0.23 ± 0.03 | 0.38 ± 0.04 | 0.02 ± 0.00 | |
| t-test | ns | ns | ns | ns | ns | ns | ns |
Nitrogen use efficiency and PCA analysis
Nitrogen use efficiency (NUE) was evaluated for barley and oat plants (Table 6). For barley, NUE was greater (p < 0.05) in Sogang (47.7 ± 2.0 kg kg-1) compared to Jaeanchal (33.2 ± 5.5 kg kg-1), however, other NUE indices such as nitrogen uptake efficiency (NUpE), nitrogen utilization efficiency (NUtE), biomass production nitrogen use efficiency (BNUE) and nitrogen harvest index (NHI) were not significantly differed. The score of NHI showed 0.49 - 0.52, which means that 50% of total absorbed nitrogen was accumulated in spike. For oat, NUtE and NHI were differed from varieties, and Joyang indicated higher score (41.5 ± 5.6 kg kg-1 for NUtE; 0.49 ± 0.01 for NHI) compared to Daeyang (24.2 ± 3.5 kg kg-1 for NUtE; 0.33 ± 0.02 for NHI). Despite of greater NUE (63.9 ± 10.6 kg kg-1) in Joyang, NUE was not significantly differed between both varieties. The association between all physiological parameters were estimated through principal component analysis (PCA) (Fig. 3, Barley; Fig. 4, Oat). In barley, PC1 and PC2 accounted for 45.2% and 24.1% of total variations at heading stage, and 50.8% and 22.8% of total variations at harvest stage, respectively. In general, the parameters represented by adjacent or parallel vectors show a strong positive association between each other. The NUE was strongly associated with dry weight (spike and shoot) and negatively related to proline (Fig. 3B). Additionally, Jaeanchal and Sogang were closely associated with osmo-protectants and NUE indices among variables, respectively. In oat, PC1 and PC2 accounted for 72.8% and 15.9% of total variations at heading stage, and 69.5% and 24.2% of total variations at harvest stage, respectively. The NUE was strongly associated with dry weight (spike) and negatively related to proline and soluble sugars (Fig. 4B). Daeyang and Joyang were greatly tied with osmo-protectants and NUE indices among variables, respectively. Overall, the current study revealed that Sogang (barley) and Joyang (oat) represented a significant effect on biomass production and NUE, and NUE was negatively associated with osmo-protectants, proline and soluble sugars. The results imply that the increase in ear yield attributes to higher N availability, suggesting that Sogang and Joyang are relatively suitable varieties in the reclaimed soil.
Table 6.
Nitrogen use efficiencies of cereal crop plants, barley and oat, grown under the reclaimed soil at harvest stage.
| Species | Variety |
NUE† (kg kg-1) |
NUpE (kg kg-1) |
NUtE (kg kg-1) |
BNUE (kg kg-1) | NHI |
|
Barley (Hordeum vulgare) | Jaeanchal | 33.2 ± 5.5 | 1.1 ± 0.3 | 31.3 ± 3.9 | 73.8 ± 13.7 | 0.45 ± 0.02 |
| Sogang | 47.7 ± 2.0 | 1.3 ± 0.2 | 38.3 ± 7.9 | 98.1 ± 3.9 | 0.49 ± 0.00 | |
| t-test | *‡ | ns | ns | ns | ns | |
|
Oat (Avena sativa) | Daeyang | 45.2 ± 4.6 | 1.9 ± 0.3 | 24.2 ± 3.5 | 136.3 ± 18.0 | 0.33 ± 0.02 |
| Joyang | 63.9 ± 10.6 | 1.5 ± 0.1 | 41.5 ± 5.6 | 131.6 ± 21.6 | 0.49 ± 0.01 | |
| t-test | ns | ns | * | ns | ** |
†Nitrogen use efficiency (NUE, Yield (ears)/Applied N), Nitrogen uptake efficiency (NUpE, Absorbed N/Applied N), Nitrogen utilization efficiency (NUtE, Yield/Absorbed N), Biomass nitrogen use efficiency (BNUE, total N accumulation in above- and below-ground biomass/Applied N), Nitrogen harvest index (NHI, NUE/BNUE).
Conclusions
In this study, multiple assessments were employed to reveal physiological responses in cereal crops, barley and oat, grown in the reclaimed soil condition. Due to low water and nutrients holding capacity, it is carefully suggested that physical properties of the reclaimed soil like Saemangeum should be priorly amended rather than chemistry. The greater spike yield varieties, Sogang (barley) and Joyang (oat), were positively associated the higher N availability expressed as NUEs, in contrast, produced lower levels of osmo-protectants such as proline and soluble sugars. These results are believed that the reclaimed land partly provides a possibility of the cultivation of barley and oat crops. Nevertheless, the detailed evaluation on salt sensitivity should be required to select the suitable ones from diverse barley and oat varieties. Currently, further experiment is being performed to understand the negative relation between greater biomass-producing variety and osmo-protectants metabolisms, the relative expression of selected proline- and trehalose-biosynthetic genes.