Introduction
Materials and Methods
Bacterial strain and culture conditions
IAA production
Ammonia production
Pot experiment
Statistical analysis
Results and Discussion
Qualitative and quantitative determination of IAA production by B. subtilis PE7
Ammonia production by B. subtilis PE7
Effect of B. subtilis PE7 culture on growth of tomato
Conclusions
Introduction
Tomato (Solanum lycopersicum L.), a member of the Solanaceae family is the second most important vegetable crop after potato due to its economic significance with a production of 189.1 million tons on the area of 5.2 million hectares worldwide (FAOSTAT, 2021). Moreover, tomatoes are natural origins of health-promoting compounds including vitamin B, lycopene, carotenoids, ascorbic acid, and polyphenolic compounds which are representative of anti-tumor, anti-oxidant and anti-inflammatory activities (Quinet et al., 2019). However, the demand for improvement of fruit quality and large comsumption of tomatoes lead to the over-application of synthetic fertilizers contributing to deleterious effects on ecosystems including soil acidification, groundwater contamination and an increase in greenhouse gas level. As a consequence, research has been focused on sustainable agricultural methods through applications of different kinds of biofertilizers which are usually based on plant growth promoting bacteria (PGPB). PGPB including species of Bacillus, Pseudomonas, Enterobacter, Klebsiella, Azotobacter, Clostridium, Azospirillium,and Serratia have been studied and utilized as biofertilizers for decades (Esitken et al., 2010; Minuț et al., 2022). Among these strains, Bacillus species become more attractive due to their endurance by developing stress-resistant endospores under unfavorable environmental conditions leading to the successful field performance. Like other PGPB, Bacillus species assist plant growth directly by facilitating nutrient efficiency via production of ammonia (NH3), solubilizing insoluble phosphate and releasing phytohormones such as indole-3-acetic-acid (IAA) and gibberellic acid (GA3) (Glick, 2012; Gohil et al., 2022). Indirectly, they reduce the degree of plant stress hormone, ethylene through production of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and induce the plant defense system by elevating the level of the defense-related compounds in response to pathogen attacks (Glick, 2012; Shin et al., 2016). Moreover, these species also act as biocontrol agents and play a significant role in inhibition of phytopathogens via secretion of diverse active compounds including antibiotics, volatile organic compounds (VOCs), hydrogen cyanide (HCN), and siderophores (Olanrewaju et al., 2017; Lee et al., 2021; Hwang et al., 2022; Kim et al., 2022).
Generally, the group of Bacillus species are ubiquitous in a broad-spectrum of environments such as air, soil, water and rhizosphere, and they are also common habitants of fermented foods such as yogurt, cheonggukjang (extra-fermented bean paste from Korea) and kimchi (Lee et al., 2013; Kimura and Yokoyama, 2019; Luo et al., 2023). Kimchi, a traditional fermented Korean side-dish prepared with Chinese cabbage, red pepper, radish and garlic, is a resource of beneficial microbes involving Bacillus species. In many reports, thebacterial species isolated from kimchi possessed unique properties with stability at a wide range of pH and temperature (Lee et al., 2013; Radhakrishnan and Lee, 2016; Jeon et al., 2017; Han et al., 2023).
The hypothesis was that the broth culture of Bacillus subtilis PE7 isolated from kimchi will promote the growth of tomato as well. In our study, the bacterial strain, B. subtilis PE7 was used to evaluate its plant growth promoting (PGP) potential on tomato plants. Therefore, the objectives of this study were to determine ammonia and IAA production by strain PE7 under in vitro conditions and to evaluate the effect of the strain PE7 culture on the growth of tomato via pot experiment.
Materials and Methods
Bacterial strain and culture conditions
B. subtilis PE7 (KACC 92549P) was isolated from kimchi in our previous study (Han et al., 2023),and this bacterial strain was used for the present study. For in vitro experiments, ten microliters of original stock culture of B. subtilis PE7 was streaked on tryptone soy agar (TSA) medium to attain pure and single colonies. For pot experiment, strain PE7 was inoculated in pink-fertilizer broth (PFB) medium and incubated in a shaking incubator at 40°C for 2 days. One liter of PFB medium consists of dextrose 2.0 g; chitin powder 0.4 g; commercial fertilizer (NPK: 20-20-20) 5.6 g; CaCO3 0.3 g; K2SO4 0.4 g, MgCl2 0.2 g, and 2 mL of micronutrients.
IAA production
For screening of IAA production, B. subtilis PE7 was inoculated in tryptone soy broth (TSB) medium containing different concentrations of ⳑ-tryptophan (0, 0.25, 0.5 and 1 g L-1) and incubated in a shaking incubator at 30°C for 3 days. The broth cultures were centrifuged at 12,000 rpm and 4°C for 15 min. The supernatant (1 mL) was mixed with of Salkowski’s reagent (2 mL) in a test tube and the tubes were placed in the dark at room temperature for 30 min. The development of pink color indicates IAA secretion by B. subtilis PE7.
IAA production by B. subtilis PE7 was evaluated quantitatively using a UV spectrophotometer (Gusmiaty and Payangan, 2019). The pre-culture of strain PE7 was inoculated in a flask containing TSB medium added with 1 g L-1 of ⳑ-tryptophan and incubated in a shaker at 30°C and 130 rpm for 7 days. The broth culture was collected every day and centrifuged at 12,000 rpm and 4°C for 15 min. The resulting supernatant (1 mL) was mixed with Salkowski’s reagent (2 mL) and incubated in the dark at room temperature for 30 min. After incubation, the absorbance was read at 530 nm by a UV-spectrophotometer and the amount of IAA secreted by B. subtilis PE7 was calculated based on the standard curve of authentic IAA.
Ammonia production
Ammonia production of B. subtilis PE7 was determined following the method by Karthik et al. (2017). A single colony of strain PE7 was pre-inoculated in a flask containing 100 mL of TSB medium at 30°C for 2 days. The pre-inoculated broth culture of strain PE7 was grown in the peptone water and incubated at 30°C for 72 h. Triplicate flasks were used for the experiment. After incubation, the broth culture was collected 12 h intervals and the supernatants were obtained by centrifuging at 12,000 rpm and 4°C for 15 min. One milliliter of each resulting supernatant was mixed with 1 mL of Nessler’s reagent. The total volume was increased to 10 mL with double distilled water (DDH2O) and thoroughly mixed using a vortex mixer. Then, ammonia production by strain PE7 was measured at a wavelength of 450 nm using a UV-spectrophotometer.
Pot experiment
Two-leaf stage of tomato seedlings were obtained by sowing the seeds in a tray which contained organic bed soil, and watered every day for 2 weeks. The seedling was transplanted into the pot containing a mixture of soil, sand, and vermiculite (1:1:1, v/v/v, pH: 6.14 ± 0.11, electrical conductivity (EC): 0.57 ± 0.01 dS m-1). The experiment was designed with three treatments: (1) water as the control (Con), (2) PFB medium as the fertilizer treatment (F), and (3) B. subtilis PE7 broth culture (PE7). The experiment was conducted with three replications and five plants were used for each replication. At 7 days after transplantation, 50 mL of each treatment per week was consecutively applied to tomato plants via soil drenching twice for the first and second time-treatment and 100 mL was used twice for third and fourth time-treatment. At 3 days after the final treatment, tomato plants were carefully uprooted and rinsed gently with running tap water. The tomato growth was analyzed based on leaf number, length, fresh weight, and dry weight of the shoot and root. The shoots and roots were oven-dried at 70°C for 3 days and determined the dry weights.
Statistical analysis
The data of plant growth parameters were used to analyze by analysis of variance (ANNOVA) using statistical analysis system (SAS) software (9.4). The mean comparison was done by the least significant difference (LSD) test at p < 0.05 level.
Results and Discussion
Qualitative and quantitative determination of IAA production by B. subtilis PE7
Several studies reported that Bacillus speciesproduced different types of phytohormones including IAA to regulate plant growth (Cherif-Silini et al., 2016; Ratnaningsih et al., 2023). As shown in Fig. 1, the intensity of pink colorization indicated that IAA production of B. subtilis PE7 was ⳑ-tryptophan-concentration dependent. Moreover, the quantitative assay revealed that IAA production gradually increased from 1 day after incubation (DAI) to 6 DAI in the ⳑ-tryptophan supplemented medium, the IAA amount attained the maximum with 25.59 µg mL-1 at 6 DAI, and then declined at 7 DAI (Fig. 2). In a previous study by Wagi and Ahmed (2019), the presence of ⳑ-tryptophan in the Luria-Bertani (LB) medium induced auxin production (35.8 and 36.6 µg mL-1) by both Bacillus cereus (So3II) and B. subtilis (Mt3b). Cherif-Silini et al. (2016) also reported that 10.35 µg mL-1 of IAA was produced by B. subtilis subsp. subtilis D7 in DF salt minimal medium containing 1 g L-1 of ⳑ-tryptophan.
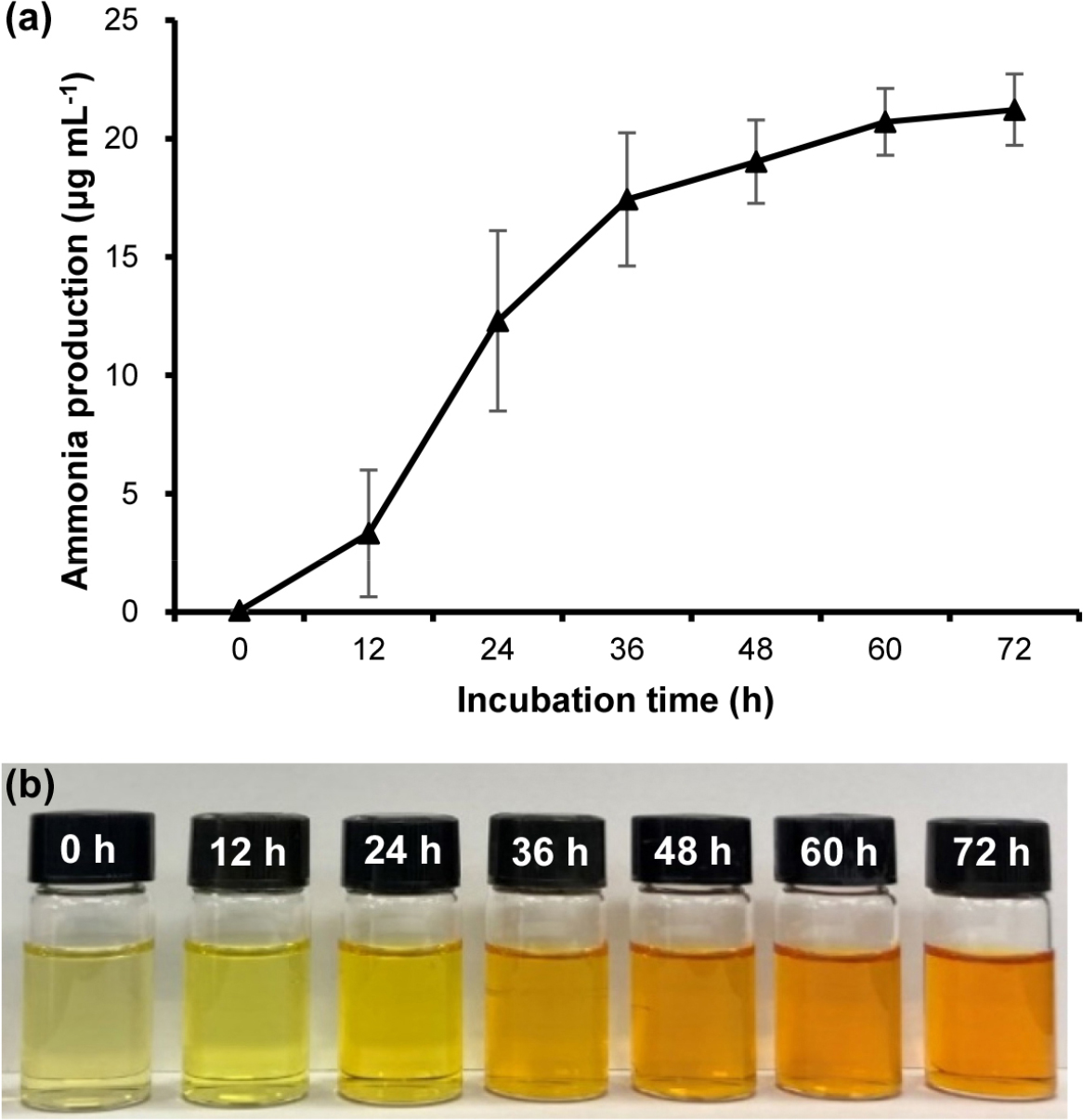
Ammonia production by B. subtilis PE7
Members of Bacillus species release ammonia not only to enhance plant growth by supplying the required nitrogen source to host plant but also to protect the plant from the attack of phytopathogens by limiting their growth (Alotaibi et al., 2022; Gohil et al., 2022). Nithyapriya et al. (2021) reported that B. subtilis LSBS2 produced 8 µg mL-1 of ammonia in peptone water after 48 h incubation period. The colorimetric assay showed that a gradual increase in ammonia production of strain PE7 within a range of 0.06 to 21.22 µg mL-1 was recorded during the experimental period in the present study. (Fig. 3).
Effect of B. subtilis PE7 culture on growth of tomato
The potential of PGPB for plant growth enhancement through direct and indirect regulation has been well documented in several reports (Katsenios et al., 2021; Zhou et al., 2021; Kouam et al., 2023). However, one of the major challenges for field application is expensive growth medium for inoculation of PGPB. Therefore, to overcome the limitation on wide application of bacterial agents for crop production, cheap fertilizer-based PFB medium was used to inoculate B. subtilis PE7 in our study. Our study demonstrated the PGP ability of B. subtilis PE7 on growth of tomato via pot experiment. As shown in Table 1 and Fig. 4, the inoculation of strain PE7 broth culture to tomato plants remarkably improved tomato growth in terms of leaf number, shoot length, fresh and dry weights of shoot as compared to the Con and F treatment. Although, there was no statistical difference of fresh and dry weights of root between PE7 and F treatments, the values of these parameters in PE7 treatment were higher than those of F treatment. The strain PE7 culture remarkably affected tomato growth resulting in a significant increase in leaf number, length, fresh and dry weights of shoot respectively as compared to the control. Although the presence of IAA and ammonia in PFB medium was not verified in this study, the growth promotion of tomato plants could be related with the action of various metabolites which accumulated in the broth culture of strain PE7. Moreover, the viable cells of strain PE7 in the broth culture might also partially involve in growth promotion of tomato plants because our in vitro IAA and ammonia assays revealed that strain PE7 possessed the capacity to produce IAA and ammonia. Bacterial IAA plays a major role in root stimulation, cell division and enlargement, and enhances root development including root elongation and abundant formation of root hairs, thereby improving water and nutrient acquisition by plant root (Bessai et al., 2022; Castillo-Alfonso et al., 2022). In addition, ammonia released by PGPB induces a direct stimulation of plant growth through nitrogen accumulation for shoot and root growth, and biomass production (Kouam et al., 2023). Various PGP properties of strain PE7 such as phosphate and zinc solubilization, siderophore and exopolysaccharide production, have been reported in our previous study (Han et al., 2023). Our results suggest that the simultaneous action of viable cells of strain PE7 and active metabolites in the broth culture were mainly responsible for achieving higher growth parameters of tomato. Ahmed et al. (2022) reported the dual effects of Bacillus amyloliquefaciens WS-10 broth culture grown in LB medium on reduction in disease incidence of tobacco bacterial wilt and an increase in dry matter contents of roots, stems, and leaves compared to the control. Moreover, the use of 100-fold-diluted broth cultures of B. subtilis and B. amyloliquefaciens which were inoculated in TSB medium, increased fruit weight of tomato plant by 30.70% and 28.81%, as compared to the control under field conditions (Katsenios et al., 2021). In a report by Qiao et al. (2017), seed treatment with the cell suspension of B. subtilis PTS-394 between OD600 value of 0.01 and 1.0 (~5 × 107 CFU mL-1), increased the fresh weight of tomato seedlings in MS agar medium. Among the treatments, the inoculation of cell suspension (~5 × 107 CFU mL-1) resulted in the greater value of fresh weight of tomato seedlings (18.36%) compared to the control. Similar growth enhancement of tomato plants by the applied cell suspension of B. subtilis PTS-394 was also demonstrated in their pot experiment. Also, in our pot experiment, the viable cells of strain PE7 (which have the capacities of IAA and ammonia production) might activate in the soil, thereby promoting tomato plants. Therefore, the viable cells of strain PE7 and its accumulated active metabolites could be considered as eco-friendly effective substitutes for chemical fertilizer to not only enhance tomato growth but also reduce the frequency of fertilizer application in sustainable agriculture.
Table 1.
Effect of Bacillus subtilis PE7 culture on growth characteristics of tomato plants.
|
Treat- ment† | Length (cm) | Fresh weight (g) | Dry weight (g) | Leaf number | |||
| Shoot | Root | Shoot | Root | Shoot | Root | ||
| Con | 9.40 ± 0.28 c‡ | 19.63 ± 5.89 a | 2.77 ± 0.08 c | 0.75 ± 0.07 b | 0.26 ± 0.00 c | 0.07 ± 0.01 b | 6.00 ± 0.00 c |
| F | 25.03 ± 1.34 b | 16.77 ± 2.54 a | 18.22 ± 0.88 b | 1.52 ± 0.26 a | 1.10 ± 0.08 b | 0.12 ± 0.03 a | 9.00 ± 0.00 b |
| PE7 | 30.30 ± 0.83 a | 19.37 ± 3.71 a | 29.25 ± 2.23 a | 1.79 ± 0.12 a | 1.67 ± 0.12 a | 0.13 ± 0.01 a | 11.00 ± 0.00 a |
Conclusions
Our study revealed that the plant beneficial bacterium, B. subtilis PE7 isolated from kimchi produced IAA and ammonia under in vitro conditions, and significantly enhanced tomato growth under in vivo conditions. Further studies such as investigation of antimicrobial activity of strain PE7 against phytopathogens, observation of its biocontrol effect on tomato diseases and evaluation of effect of strain PE7 culture on tomato growth under field conditions should be carried out. Based on our results, the application of B. subtilis PE7 culture through inoculation in fertilizer-based medium could be used as a potential eco-friendly approach for plant growth promotion to reduce frequent use of synthetic fertilizer in crop production.