Introduction
Various weeds in persimmon orchards in South Korea are frequently mowed from spring to autumn to prevent excessive growth, and their residues are left on the soil surface, acting as a green manure. The growing weeds, competing with crops for soil nutrients, can take up 232 kg N, 42 kg P, and 267 kg K per ha from the soil during the year in a South Korean orchard (Lim et al., 2012). However, weed residues can contribute to improving the organic matter supply in the orchard soil (Choi et al., 2011). Lim et al. (2012) reported that the dry matter (DM) of weeds mowed throughout the year reached 15 ton per ha in a pear orchard. When the residues of weeds or cover crops are deposited onto the soil surface after mowing, mineral nutrients from the residues return to the soil through decomposition and these can be used in crop growth (Talgre et al., 2014; Jahanzad et al., 2016). The cycle of nutrients after mowing weeds could play an important role in nutrient supply for maintaining the productivity of persimmon orchards.
The nutritional value of cover plants is closely related to the amount of nutrients in the residues and their releasing patterns subsequent to decomposition (Valenzuela-Solano and Crohn, 2006; Murungu et al., 2010). Decomposition and nutrient release of the residue are affected by the chemical properties of the residue and many environment factors including moisture, temperature, soil minerals and acidity, and microbial activity (Trinsoutrot et al., 2000; Valenzuela-Solano and Crohn, 2006; Matos et al., 2011; Ferreira et al., 2014). Mineralization of organic matters in the soil is negatively correlated with the carbon-to-nitrogen (C/N) ratio (Im et al., 2017). Therefore, Gramineae residues with high C/N ratios decompose slowly, later releasing nutrients into the soil, when compared with that of legume residues with low C/N ratios (Ferreira et al., 2014). Lindsey et al. (2013) reported that the weed residue with a C/N ratio over 19 could release 25% to 45% of the total N within 2 weeks. Residues of clover green manure on the soil surface released about 90% of the initial K during the first 10 weeks after residue placement, while wheat residue released about 70% of K (Lupwayi et al., 2006). Burying the residues of sod into the soil accelerates decomposition and nutrient release more than placing them on the soil surface, as a larger surface area is exposed to microbial activity (Wilson and Hargrove, 1986; Seo et al., 1998; Jahanzad et al., 2016). Clover residue under tillage released up to 70% of the residue P after 12 months, which was higher than that under no tillage condition (Lupwayi et al., 2004). In Nordic conditions, 80% of P and 89% of K from legume shoot residue were released during the first 6 months after being incorporated into the soil (Talgre et al., 2014).
Dominant weed species on the floor of persimmon orchards seasonally change across different environments, leaving a great deal of organic matter containing various minerals on the soil surface after mowing. Therefore, these managing mineral nutrients for the benefit of persimmon trees should be based on the understanding of the patterns of nutrient release from the weed residues. Unlike cover crop residues, very little information is available on nutrient release from weed residues under the orchard conditions. In our previous study in the persimmon orchard (Choi et al., 2017), green manure residue mixed with various weeds in spring decreased by 63 - 71% after 2 months of litterbag deposition on the soil surface, and the residue released 51 - 67% of N, 54 - 55% of P, and 92 - 94% of K during the time. However, the release of mineral nutrients from green manures of dominant spring weeds in the orchard has not been evaluated. The present study was conducted to determine the seasonal residue decomposition and nutrient release after mowing two dominant spring weed species at a persimmon orchard.
Materials and Methods
Research site
This study was conducted from May 2011 to June 2013 at a persimmon orchard in the Sweet Persimmon Research Institute (35°16'57"N; 128°43'6"E), located in Gimhae, South Korea. When the experiment was initiated in 2011, the 17-year-old ‘Fuyu’ persimmon (Diospyros kaki) trees had been grown at a density of 6 × 6 m and trained into open-center form. The rainfall and temperature data during the experiment were obtained from automatic weather station located at the Research Institute. Annual rainfall and mean temperature are shown in Table 1. Rainfall was characterized by the highest in 2012 and the lowest in 2013. Soil type on the experimental site exhibited characteristic of sandy-loamy Hydragric Anthrosols. The soil sample, collected from the 0 - 20 cm layer on May 12, 2011, contained 7.7 g kg-1 organic matter, 6.7 pH in water, 0.33 mg kg-1 total N (Kjeldahl), 176 mg kg-1 available P2O5, and 0.52 cmolc kg-1 exchangeable K. When rainfall was low in the growing season, irrigation was provided to the trees via drip emitters.
Table 1. Monthly average temperature and rainfall during the experiment in the persimmon orchard.
Materials and litterbag deposition
Two weed species, Elymus tsukushiensis var. transiens and Vicia hirsuta, were used as the experimental materials, which are common grass (Gramineae) and legume (Leguminosae) species, respectively, naturally growing at spring persimmon orchards in South Korea. They were cut at about 3 cm above ground level at the orchard on May 13 in both 2011 and 2012 when the sampling corresponded to general spring mowing time. At the sampling times, E. tsukushiensis var. transiens had begun to grow ears and V. hirsuta was flowering. To simulate the fate of the two weeds on the soil after mowing, the litterbag deposition was designed as described in previous studies (Seo et al., 1998; Talgre et al., 2014; Jahanzad et al., 2016; Choi et al., 2017). The litterbags were made of 60-mesh nylon fabric with dimensions of 50 × 25 cm.
The mowed weeds were immediately chopped to about 10 cm in length, and 350 g of the fresh samples was placed into each litterbag. Three litter bag samples of each weed species were used to determine the initial dry weight (DW) of weeds and nutrients within a litterbag. About 15 litter bags per tree were deposited on the ground within a 2-m periphery from the tree trunk. Three tree replications per weed species were assigned for the litterbag depositions in both years. The bags were placed away from the drip emitters and fixed with iron bars to prevent them from being moved by the wind. Weeds grown between the bags after deposition were cut to about 5-cm above the ground level on July 2 and September 2 in both years. Compost and fertilizers were not applied to the deposit sites during the experiment to minimize any external influences on weed decomposition.
Sampling and measurement
Three litterbags containing each weed species were collected from the three deposit sites at one month intervals from the deposition day until November 13 the mowing year and on March 13 and June 13 the next year. The weed residues were taken out of litterbags and roots and stems, adhering to the residue, were removed. The residues were oven-dried to a constant weight at 80°C to calculate the ratio of DM remaining after the deposition. The dried samples were ground through a 20-mesh screen for determining N, P, and K. The C/N ratio was measured by the dry combustion method on a CNS analyzer (Vario-Max, Elementar, Hanau, Germany) for the initial sample on May 13 in both years. To determine total N, subsample of 0.2-g residue were analyzed using a Kjeldahl instrument (Kjeltec 2300, Foss Co., Hgans, Sweden) by using the micro-Kjeldahl method (Nelson and Sommers, 1973). After digesting a 0.5-g sample with HClO4 and H2SO4 on a heating block according to NIAST (2000), P and K were analyzed by an inductively coupled plasma emission spectrometer (ICPS-7510, Shimadzu Co., Tokyo, Japan). The ratios of nutrient content remaining until sampling dates after litterbag deposition were calculated by recording the weed DW and the concentrations of N, P, and K at each sampling date.
Data analysis
Mean and standard deviation (SD) values were calculated based on the data obtained from the residues in three litterbags for each weed species at every sampling date. The statistical software package SigmaPlot program (Version 8.0 SPSS Inc. USA) was used to analyze the data.
Results
Table 2 shows C/N ratio and nutrient concentration in DMs of two weed species before litterbag deposition on May 13, 2011 and 2012. E. tsukushiensis var. transiens had higher C/N ratio and lower N concentrations than V. hirsuta. The C/N ratio was 14.6 in 2011 and 23.7 in 2012 for E. tsukushiensis var. transiens while the ratio decreased to 11.0 and 9.8 for V. hirsuta, respectively. N concentration was 24% and 82% higher in V. hirsuta in 2011 and 2012, respectively, compared with 2.98% and 2.26% of E. tsukushiensis var. transiens. There were no consistent differences in P and K concentrations between the two weed species.
Table 2. C/N ratio and nutrient concentrations in dry matter of two weed species mowed under tree canopy of a persimmon orchard on May13 in 2011 and 2012.
‡NS, *, **Non-significant or significant at P = 0.05 or 0.01 by Student’s t-test, respectively.
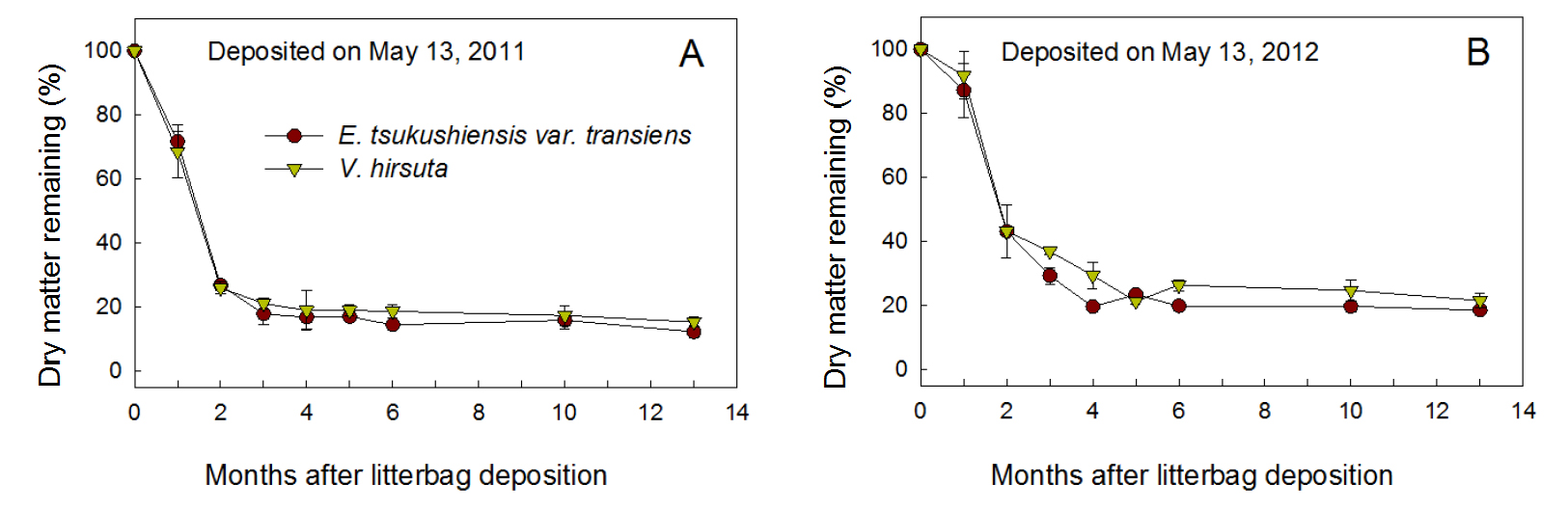
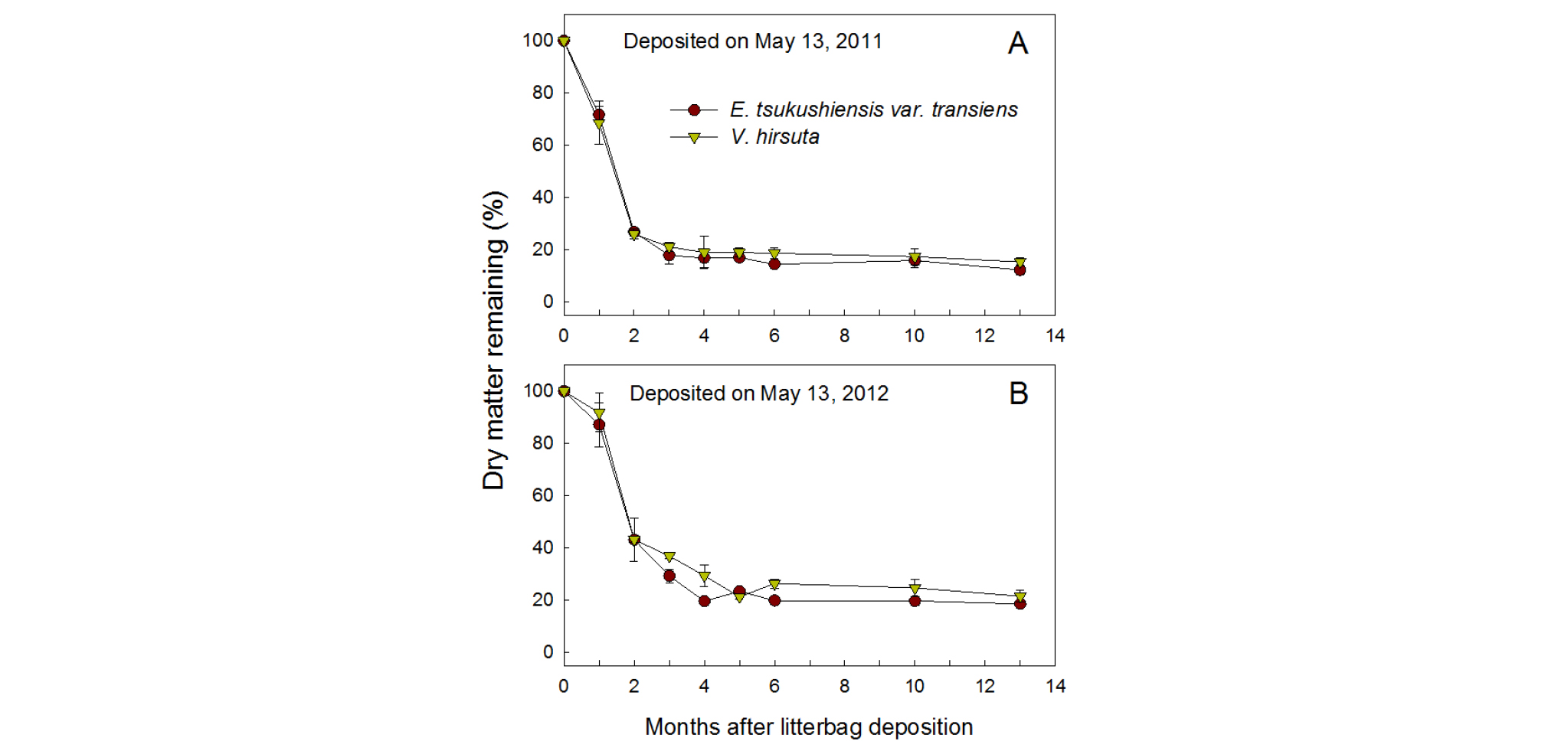
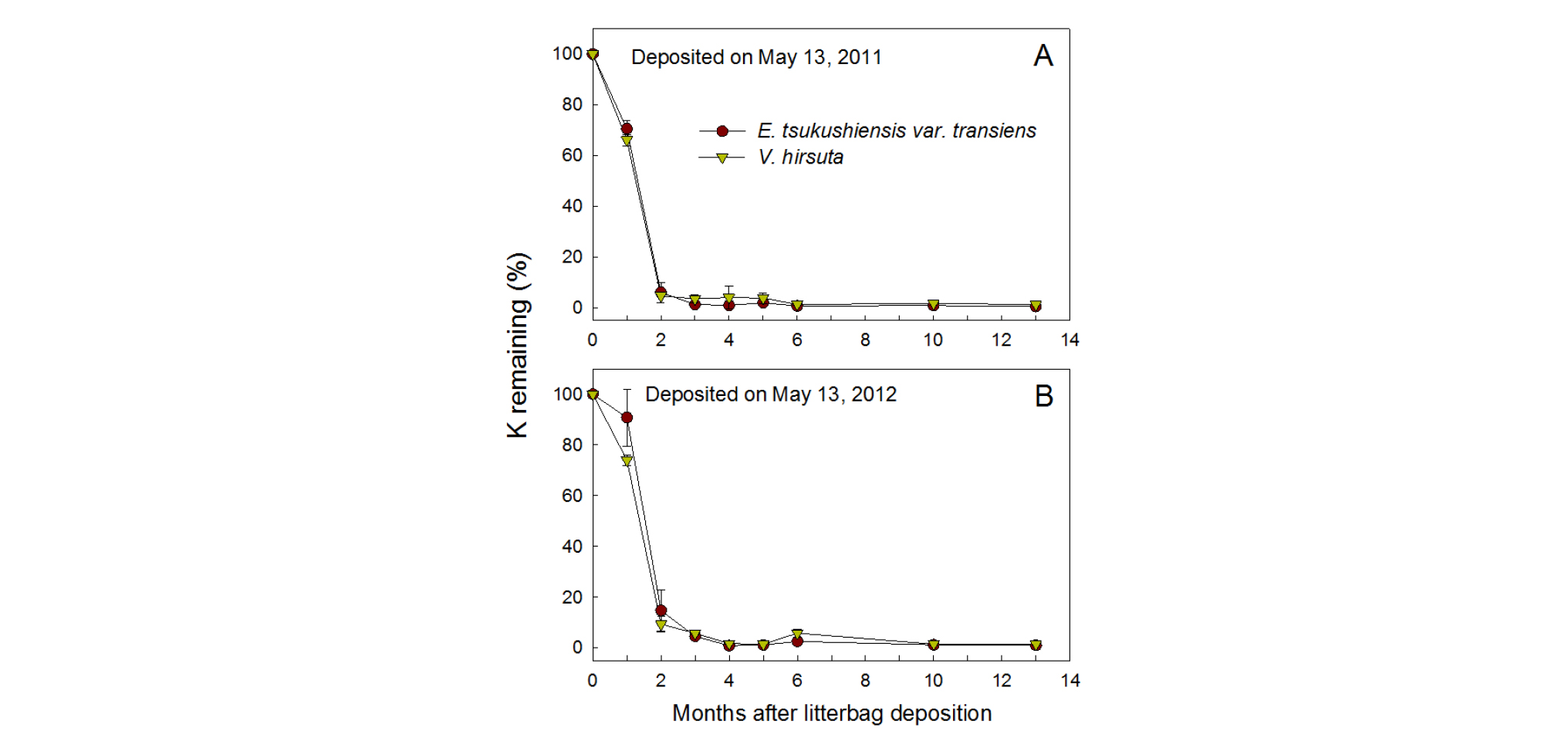
Fig. 1 shows that weed residues lost most of their DM through decomposition during the first 2 months spent under the persimmon tree canopies. In 2011, for both weed species, DM remaining (% of initial DM) decreased to 69 - 72% during the first month and to 26 - 27% at 2 months (July 13), and then it gradually decreased to under 17 - 19% after 5 months (October 13). Differences in the weed species did not clearly affect the pattern of residue decomposition. In 2012, the residues of the two weed species decomposed more slowly than those in 2011 during the first three months. In 2012, regardless of the weed species, DM remaining (% of initial DM) decreased to 87 - 92% during the first month, 43% after 2 months, and then it gradually decreased to 22 - 23% after 5 months. Residues of both weed species still remained at 12 - 15% and 19 - 22% of the initial DM, respectively, until 13 months (June 13 in the following year) after the 2011 and 2012 depositions.
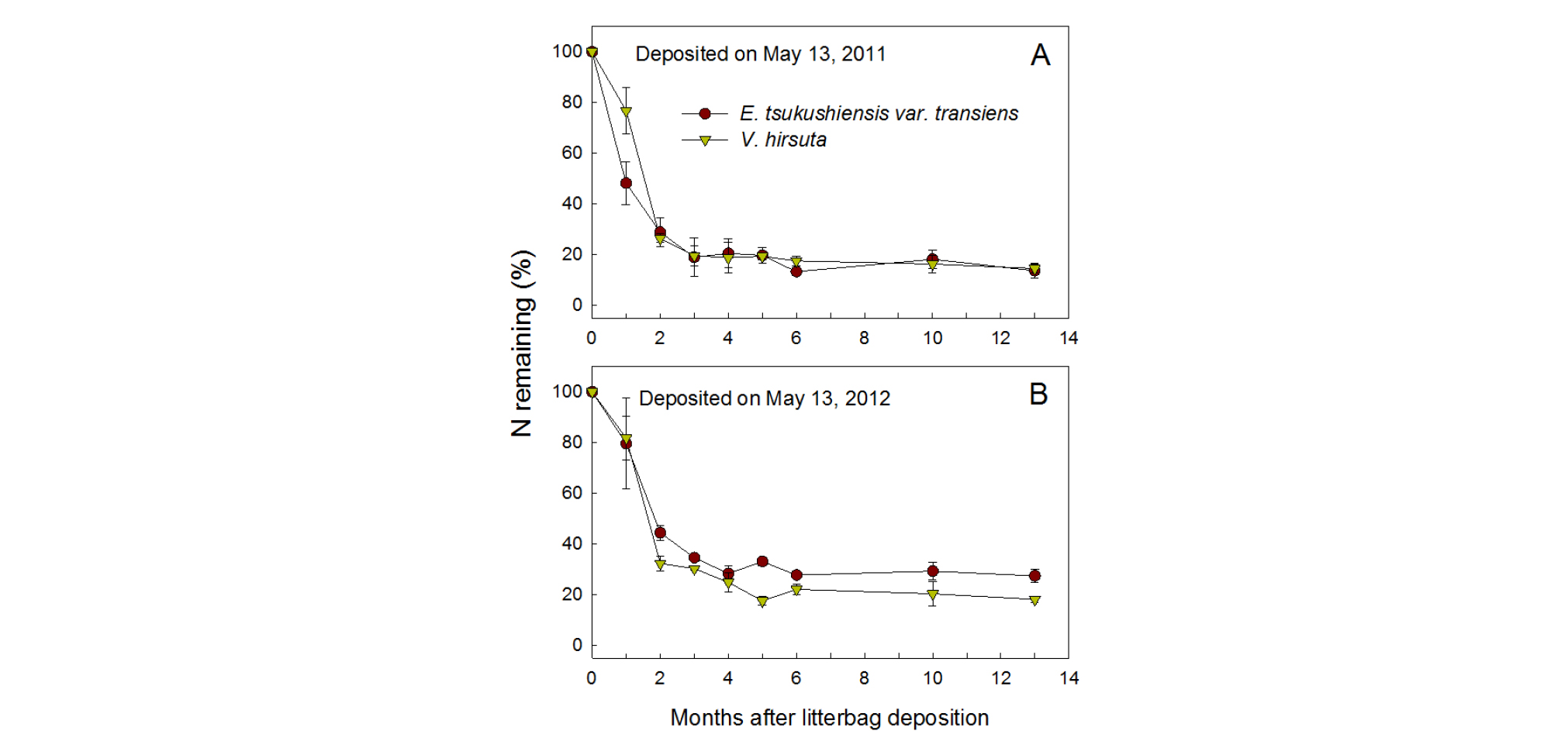
Overall, nutrients released from the residue followed the pattern of decomposition, but it differed slightly depending on the deposition year and type of nutrient (Figs. 2, 3, and 4). Nutrient releases were faster during the first two months in the 2011 than 2012 samples, without any apparent changes by different weed species. N remaining (% of initial content) in the residues of both weed species decreased to 48 - 77% during the first month and 26 - 29% after 2 months in 2011, while the ratio decreased to 80 - 82% and 32 - 44% during the respective times in 2012 (Fig. 2). After 4 months, N remained under 21% in 2011 and under 25% in 2012.
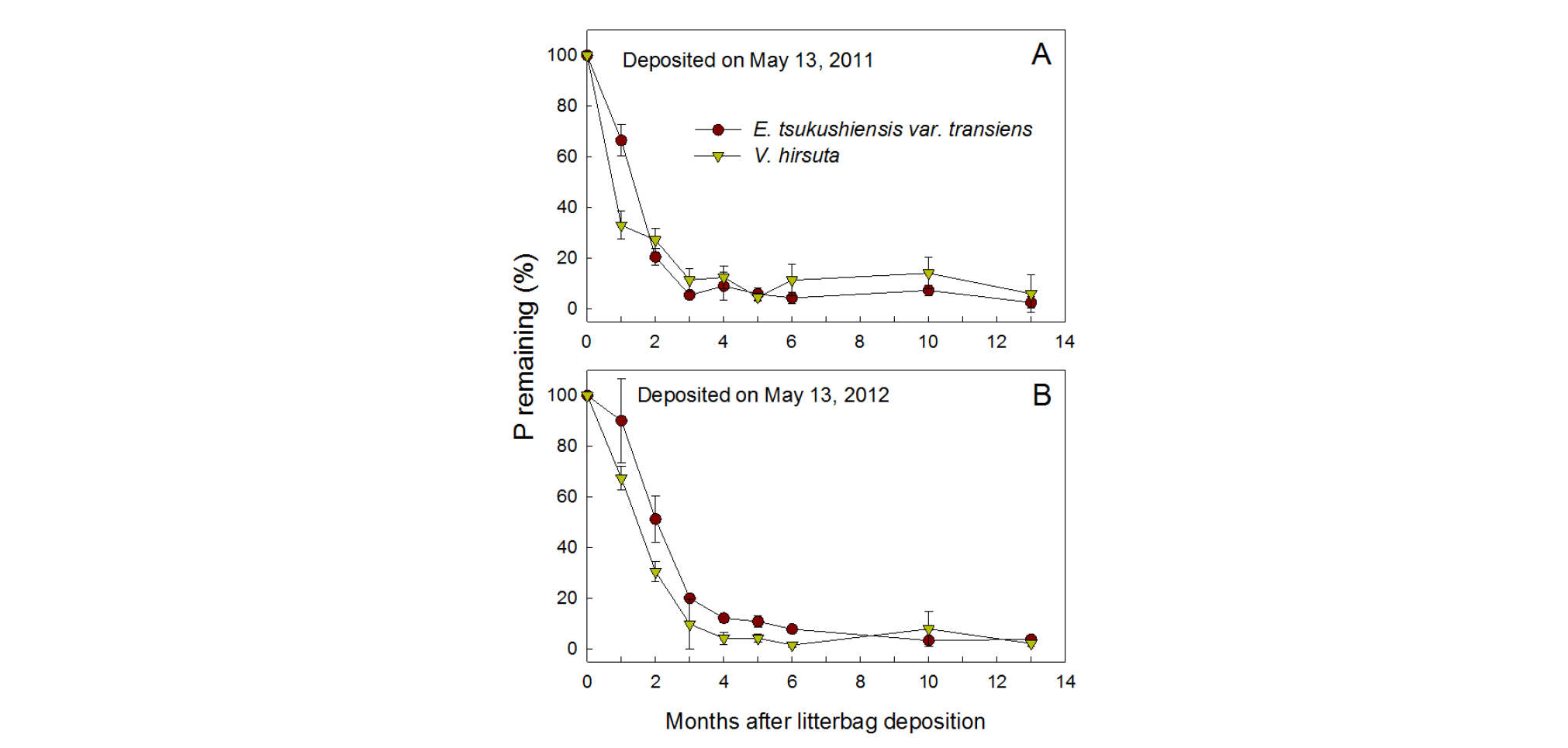
At the beginning of decomposition, P was released slightly faster from residues of V. hirsuta than E. tsukushiensis var. transiens, but this release was not as fast as the N released (Fig. 3). During the first month, P remaining decreased to 66 - 90% in E. tsukushiensis var. transiens and 33 - 67% in V. hirsuta in both years, showing faster decline in 2012 than 2011. The ratio decreased to 21 - 51% and 27 - 31% after 2 months in the respective weed species. P was mostly released after 16 months in both year weeds, when the remaining P was only 0.6 - 2.3% of its initial content.
The most drastic release among the different nutrients was found for K (Fig. 4). For both weed species, K decreased to 66 - 71% during the first month and the ratio rapidly decreased to 4.6 - 6.0% after 2 months of deposition in 2011, and to 74 - 91% and to 9.4 - 14.7%, respectively, in 2012, with a slightly faster release in V. hirsuta. Since 6 months, the ratio gradually stabilized to 0.4 - 1.5% regardless of weed species and year of sampling.
Discussion
A lower C/N ratio and higher N concentration in V. hirsuta may be attributed to N fixation by this legume weed. The rapid DM loss observed up to 2 months after deposition could be related to high temperatures and rainfall during the summer months, since rising air temperatures and rainfall promote decomposition of organic residues (Agehara and Warncke, 2005; Cabrera et al., 2005; Ferreira et al., 2014). High temperatures and rainfall after residue deposition have been known to affect microbial activity and the removal of soluble compounds, thereby increasing the decomposition process and causing reduction in the organic residues (Musvoto et al., 2000). Therefore, higher rainfall in 2011 than 2012 (Table 1) might have caused faster decomposition during the four months after litterbag deposition in 2011. In general, a high C/N ratio and low N concentration of plant residues delay decomposition (Baijukya et al., 2006; Lindsey et al., 2013) with slow mineralization (Im et al, 2017). In this study, although the C/N ratio and N concentration of DM were significantly different between the two weed species, the difference did not change the seasonal decomposition rate of the green manures. These results reflect that environmental factors including temperature and rainfall influenced the residue decomposition more than the C/N ratio and N concentration of the weeds. Slow decomposition in the following year after deposition may be the result of a high lignin content remaining in plant residues for an extended period of time (Silver and Miya, 2011).
A similar pattern of nutrient release from green manures has been observed in the previous studies (Ferreira et al., 2014; Choi et al., 2017), although the plant species were different, indicating that the mineralization process depends on the decomposition rate (Talgre et al., 2012). Changes in nutrient release from weed residues in this study differed slightly from those of other cover plant residues (Seo et al., 1998; Lupwayi et al., 2006; Jahanzad et al., 2016). This difference could be ascribed to different chemical, physical, and biotic factors of the organic residues under different environments (Trinsoutrot et al., 2000; Matos et al., 2011; Ferreira et al., 2014). The faster loss of nutrients during the first two months of residue decomposition in 2011 than 2012 might be the result of a higher rainfall in 2011, since rainfall promotes the release of soluble nutrients from plant residues (Lupwayi et al., 2006).
Due to similar environment, nutrient releases in this study were consistent with results of our previous study that residue, mixed with several weed species, released mostly N, P, and K for 2 months after litterbag deposition (Choi et al., 2017). As previously reported for organic substrates (Jalali and Ranjbar, 2009; Ranjbar and Jalali, 2012), N release followed the residue decomposition more closely than P and K releases. P in the initial stage of decomposition could be released easily from soluble P in the residues and then slowly from mineralization of organic P (Ha et al., 2007). Jones and Bromfield (1969) demonstrated that 40 - 60% of total P in grasses and legumes was present as inorganic P, being rapidly released after incorporation of the residues into the soil. On the other hand, organic P compounds like phytin might continuously release extra P from the residue (Lupwayi et al., 2007).
The fastest release of K among the residual nutrients indicated that K is not associated with structural components of plants and normally exist as a soluble form in the plant residues (Marshner, 1995; Ranjbar and Jalali, 2012). K release has also been known to depend less on microbiological decomposition of the residues, compared with N or P (Marschner, 1995; Lupwayi et al., 2006; Talgre, 2012). Therefore, K release could continue from residues of weeds mown in autumn even during winter when decomposition could not be active (Choi et al., 2017).
Since organic residues release its mineral nutrient to the soil, the nutrient cycling may directly determine the nutrient uptake of subsequent crops (Soon and Arshad, 2002; Talgre et al., 2012). During summer, persimmon trees might uptake mineral nutrients from the soil where residues of weeds mowed in May release their mineral nutrients. A total of 130 kg N and 100 kg K per ha is recommended as supplemental fertilizers for persimmon during the growing season in South Korea (RDA, 2013). How much of nutrients from the weed residues can be used for succeeding growth of persimmon trees depends on total amount of nutrients in weeds mowed per unit land and on the nutrient release rate during the decomposition. Also, availability of the nutrients for the trees may be affected by the amount of newly growing weeds which again take up nutrients from the soil. Our results indicated that when weeds are mowed in May, adjusting time and amount of supplemental fertilizer application for persimmon orchard should be decided considering the pattern of nutrient release from weeds residues.