Introduction
Heavy metals in soils originate from various sources, such as geological weathering, mining, industrial wastes, automobile emissions, and agricultural practice (Nagajyoti et al., 2010; Li et al., 2014). Among these sources, mining is considered to be one of the most significant and important sources of heavy metal contamination (Liu et al., 2005; Acosta et al., 2011; ). Hence, agricultural soils near mining areas are often contaminated by heavy metals, due to deposition of mining dust, and inflow of aqueous runoff from acid mining drainage (Lee et al., 2001; Kim et al., 2015).
For those reasons, numerous remediation techniques have been developed, to reduce the total and/or phytoavailable heavy metal concentration in soils (Mulligan et al., 2001; Desogus et al., 2013). These include soil replacement technique, in which contaminated soil is replaced with uncontaminated soil sourced from another area, and soil washing technique (Dermont et al., 2008; Yao et al., 2012). However, these soil conventional remediation technologies are costly and have several shortcomings, such as causing secondary soil contamination and destruction of the ecosystem (Efroymson et al., 2004; Herath et al., 2017; Vithanage et al., 2017).
Therefore, the ultimate remediation techniques that are cost-effective and environmentally friendly should be developed. Also, since many contaminated soils are used for agriculture purposes, remediation techniques must be carefully designed to maintain the properties for appropriate plant growth performance. One option is stabilization (immobilization) of heavy metals in soil using soil amendments, which is an environmentally friendly and cost-effective technique (Kumpiene et al., 2008). For example, application of lime and red mud resulted in increased Cd, Cu, Ni, Pb, and Zn immobilization, and subsequently not only decreased translocation of these heavy metals to the above ground tissues of Festuca rubra, but also increased the plant biomass yield (Gray et al., 2006). However, the immobilized heavy metals still remain in the soil, and when soil conditions are changed, they may be released into soil solution again. Phytoremediation is also one of the environmentally friendly remediation techniques (Raskin et al., 1997; Lee, 2013). McGrath et al. (2006) reported that Thlaspi caerulescens grown in the Cd contaminated field soil (Cd: 7.5 mg kg-1) for 14 months removed 4.2 kg Cd ha-1 (21.6 % of the total soil Cd in the top 20 cm depth). However, the practical application of this technique has been limited by factors such as plant species, growing time, and level of contamination (Salido et al., 2003). Furthermore, the efficiency of phytoremediation depends on root length and density (Mejáre and Bülow, 2001; Salido et al., 2003). Consequently, since these remediation techniques have both advantage and disadvantage, preliminary investigation of contaminated sites, such as soil properties and the heavy metal concentrations in soil depth, is required to select the proper remediation technique introduced above, or to employ a new remediation technique according to the results of the preliminary investigation.
In addition to these conventional techniques, a novel soil reversal technique would be one other possible option, in which contaminated topsoil layer (tillage layer) is replaced with uncontaminated or less contaminated subsoil layer, through turn over of the soil layer. In particular, this technique is applicable to severely contaminated paddy field soil nearby mining area, where the surface soils are relatively more contaminated than subsoils by irrigation with acid mine drainage, mine tailings input, and fallout of dust from mining operations (Jung and Thornton, 1997; Zhou et al., 2007; Ettler et al., 2011). For example, if paddy surface soil (0 - 50 cm) is highly contaminated, while the subsoil layer (50 - 100 cm) is relatively uncontaminated, the surface soil could be reversed with the subsoil. The application of this technique can guarantee less metal uptake by rice, as rice root reached 50 - 55 cm depth below the soil surface under reversed, less contaminated soil layer (Xu et al., 2013). However, in this technique, recontamination of the reversed surface soil might occur as time passes, because of the capillary rise of contaminated water from the reversed contaminated subsoil. Therefore, additional measures may be required to prevent recontamination.
The current study was carried out to investigate the applicability and effectiveness of a novel soil reversal technique for reducing Cd and Pb uptake in rice grown in contaminated paddy field soil near abandoned mines. Also, in order to prevent recontamination by the capillary rise, the effects of the lime layer and capillary break gravel layer between the reversed surface and subsoil layers were examined.
Materials and Methods
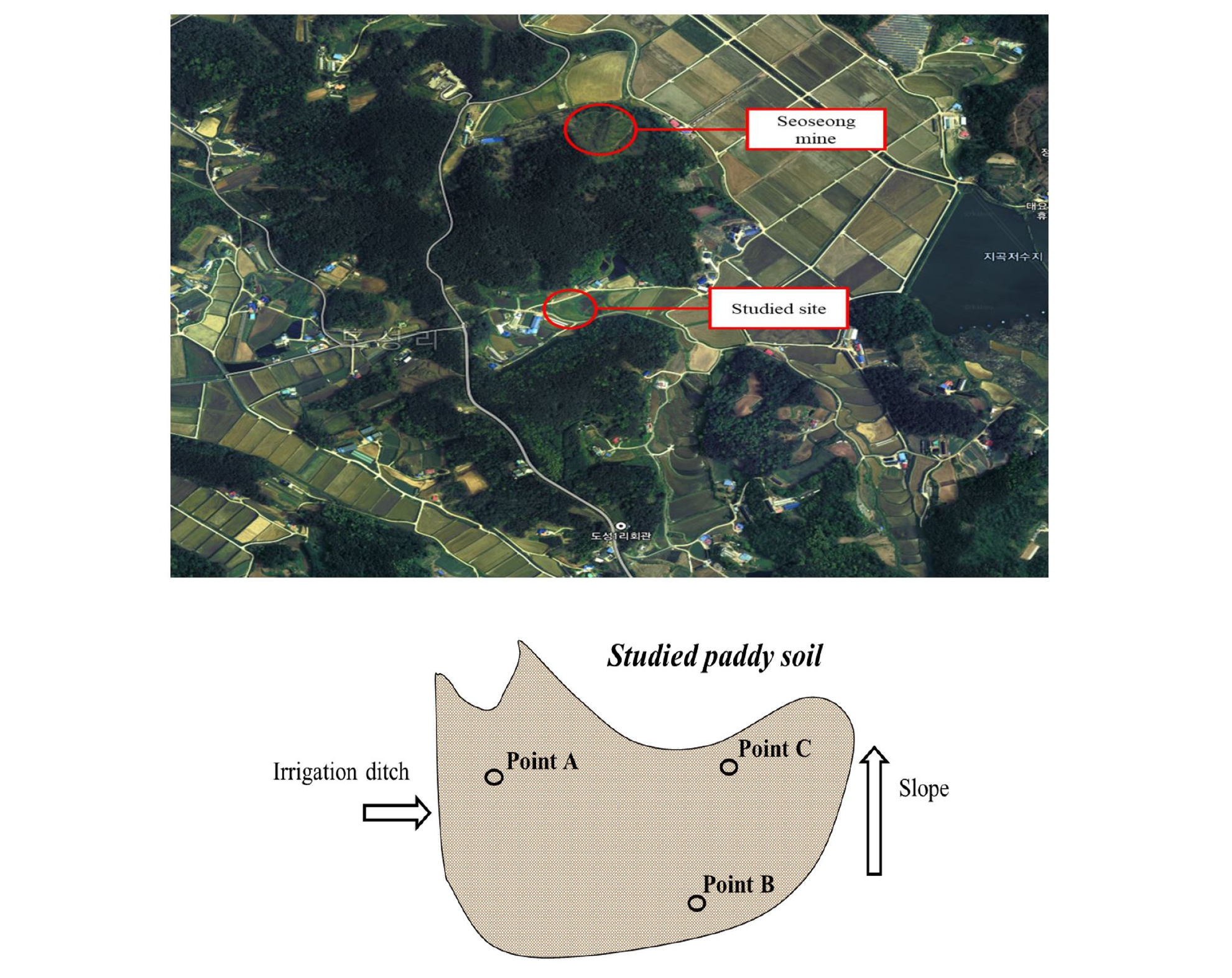
Preliminary investigation of soil properties Soil sampling: The paddy soil (0.3 ha) used for the field study is located near (< 1 km) Seosung Pb/Zn mining site (Seosan, Korea), which was abandoned in 1996. Firstly, in order to investigate the heavy metals concentrations in soil depth, 3 sampling points were selected, according to the distance from the agricultural drainage ditch, and the flow of water (Fig. 1). Then, the soil samples were collected at 20 cm steps down to 100 cm depth, using Geoprobe tools and disposable plastic liners. At that time, soil samples were also collected at 25 cm steps down to 100 cm depth using hand auger for the determination of physicochemical properties. The collected soils were air-dried and sieved < 2 mm, and stored in a plastic container for further analysis, as below.
Soil characteristics: Soil pH was measured in a 1:5 soil:distilled water suspension, using a pH meter (Model 555A, Thermo-Orion, USA) after 1 h shaking, and soil organic matter (SOM) was determined by the Walkley-Black method (Nelson and Sommers, 1996). Inorganic nitrogen content, as NH4-N and NO3-N, was determined using a QuikChem automated ion analyzer (QuikChem 6000 Series, Lachat Instruments, USA), after extraction with 2 M KCl. Available phosphorus was determined by the chlorostannous acid method (Kuo, 1996). Exchangeable cations (Ca, K, Mg, and Na) were determined using atomic absorption spectrometry (AAS, AA-6701, Shimadzu, Japan), after extraction with 1 M ammonium acetate (pH 7.0); while cation exchange capacity (CEC) was measured as the sum of the content of H+ and exchangeable cations. Soil available silicate was determined by UV-Spectrophotometry (UV-2401PC, Shimadzu, Japan) at 700 nm, after 1 M NaOAc (pH 4.0) extraction (NIAST, 2000). Soil texture was determined by the hydrometer method (NIAST, 2000). To compare with the soil safety levels suggested by the Korean Ministry of Environment, heavy metals concentrations in soils were determined using 0.1 M HCl extraction. Briefly, soil (5 g) was extracted with 0.1 M HCl (25 mL) and shaking on an end-over-end shaker for 1 h, and the Cd and Pb concentrations in the extracted solution were determined by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, Optima 3200XL, Perkin Elmer, USA).
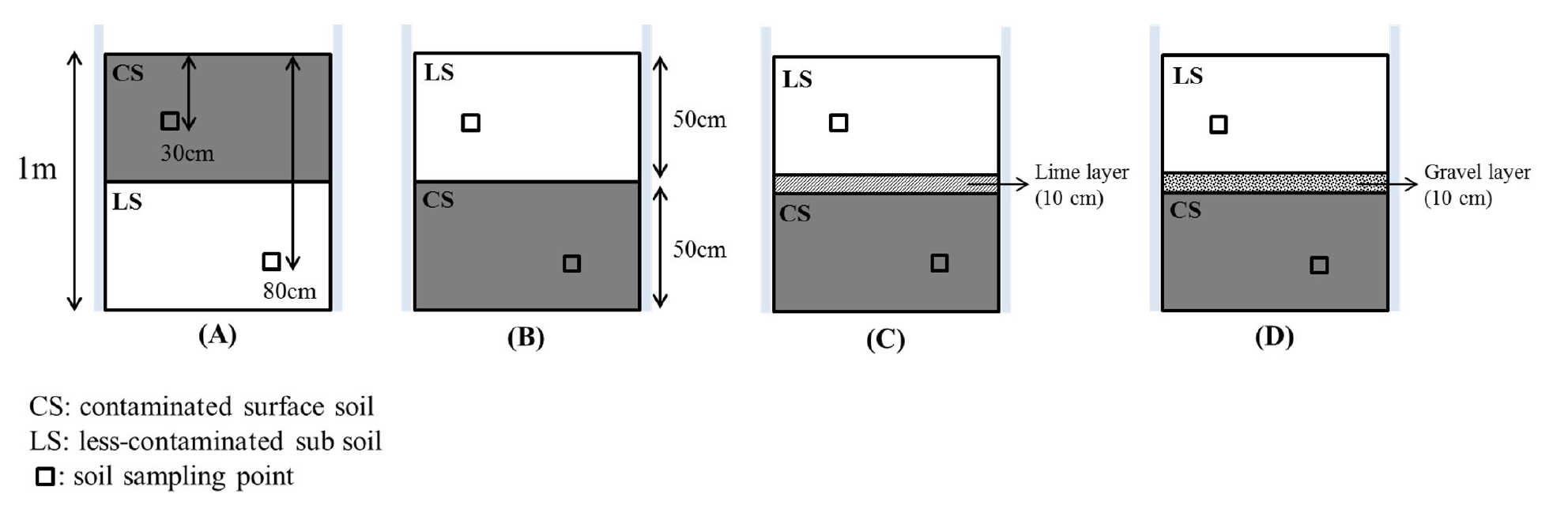
Field plot experiment After prior investigation, it was found that 0.1 M HCl extractable Cd and Pb concentrations at the 3 points showed a different trend with soil depth. it was possible to apply the soil reversal technique at point B, which was the furthest from the irrigation ditch (Fig. 1) (the detailed results are described in ‘Results and discussion’). Thus, the plots were prepared around site B in May by using plastic retaining walls (2 m × 2 m × 1 m) in soil, after removing the cultivated soil with a mechanical excavator. When excavating soil, surface soil (0 - 50 cm) and subsoil (50 - 100 cm) were carefully separated, and then each plot was uniformly refilled with separated surface soil, followed by subsoil. In order to prevent recontamination through the capillary rise of contaminated water from the reversed sublayer, lime (agricultural lime, CaCO3) layer (10 cm) plot and capillary break gravel layer (10 cm) plot were composed between the reversed surface soil and subsoil layers. For comparison, a control plot without any process was also included. Finally, a total of four plots were constructed, as follows: (1) control plot; (2) reversed soil (RS) plot; (3) reversed soil with lime break layer (RSL) plot; and (4) reversed soil with gravel break layer (RSG) plot (Fig. 2). After that, 64 rice seedlings (Oryza sativa L.) were cultivated in each plot from June to October under flooding field conditions.
In order to determine the changes in 0.1 M HCl extractable Cd and Pb concentration in each soil layer, soil samples at 30 cm and underlayer (80 cm) were collected in August and October using a hand auger and they were air-dried and sieved < 2 mm. At harvest, all rice cultivated in each plot was collected with a sickle, and the husks were removed from the rice grains. After drying at 65 °C for 48 h, the brown rice was polished using a mill. After weighing, the polished (white) rice flour obtained was homogenized, and then stored in polyethylene bags for further analysis.
Soil and plant analysis The prepared soil samples were analyzed for the 0.1 M HCl extractable Cd and Pb concentrations, using the methods described above. For the successful application of the soil reversal technique, not only reducing the level of heavy metals in soils (rhizosphere) but also maintaining the soil properties related to plant productivity was required. Soil pH and EC were the most useful and easily obtained characteristics of soil that influence plant productivity (Zhang and Wienhold, 2002Kemmitt et al., 2006). Therefore, collected soil pH and EC were also determined, using the methods described above.
The prepared rice sample (1 g) was digested with concentrated HNO3 (10 mL), diluted 100 mL using distilled water, and filtered through Whatman No. 42 filter papers, prior to the determination of Cd and Pb concentration by ICP-OES. To ensure the accuracy of ICP analysis, the digestion batch included a certified reference material (NIST 1568a, rice powder), and the average recoveries for the Cd and Pb were 87 ± 4 % and 86 ± 5 %, respectively.
Data analysis For ease of visualization, the average values, together with the standard deviation, are presented in a table and figures. Significant differences among Cd and Pb concentrations in the rice samples were analyzed by analysis of variance (ANOVA), using a SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results and Discussion
Preliminary investigation of soil properties The 0.1 M HCl extractable Cd and Pb concentrations in whole soil profile until 100 cm depth collected at points A and C exceeded the soil environment guideline values for Cd (1.5 mg kg-1) and Pb (100 mg kg-1) (Ministry of Environment, 2009). This was attributed to the irrigation water contaminated by acid mine drainage. It is widely known that a large amount of water is required in paddy cultivation. Among the preliminary investigated soil sampling sites, point A is located closest to the irrigation ditch, indicating a large amount of water contaminated with heavy metals had flowed and moved downwards through the soil profile. Also, point C is located at the lower slope position. Thus, this site is influenced by a relatively higher volume of contaminated water than point B. Consequently, the soil reversal technique was difficult to apply to points A and C, because these sites were deeply contaminated with Cd and Pb, and thus other appropriate remediation techniques should be employed at these sites.
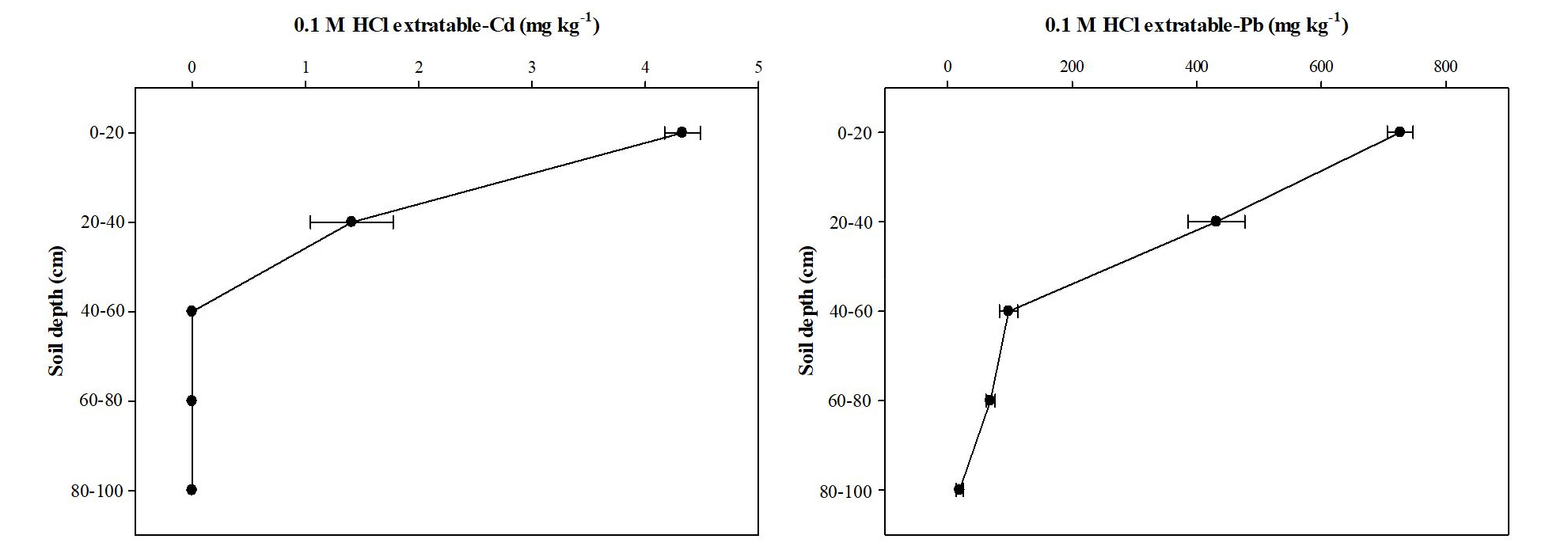
At point B, the 0.1 M HCl extractable Cd and Pb concentrations were markedly different between the cultivated layer (surface soil layer, 0 - 50 cm) and the subsoil layer (50 - 100 cm). Fig. 3 shows that 0.1 M HCl extractable Cd and Pb concentrations in the soil were 4.3 and 726.7 mg kg-1 at 0 - 20 cm, and 1.4 and 431.7 mg kg-1 at 20 - 40 cm. These values exceeded soil environment guideline values for Cd (1.5 mg kg-1) and Pb (100 mg kg-1) (Ministry of Environment, 2009). However, compared with the concentrations at the upper soil layer (0 - 40 cm), 0.1 M HCl extractable Cd concentration in the soil collected at 40 - 60 cm was below the limit of determination. Also, 0.1 M HCl extractable Pb concentrations in the soil collected at 40 - 60 cm were 86 % lower than those of the soil collected at 0 - 20 cm.
Unlike Cd and Pb concentrations, the paddy soil physicochemical properties, such as soil pH, NO3--N, exchangeable-Ca and -Mg, CEC, and texture showed similar results at all depths. However, SOM, exchangeable K, and available SiO2 in the surface soils (0 - 50 cm) were slightly higher than those in subsoils (50 - 100 cm), while NH4+-N in surface soils was relatively lower than that in subsoils (75 - 100 cm) (Table 1). This could be influenced by the fertilizer application and continued rice cultivation. Nevertheless, soil pH, exchangeable-Ca and -Mg, and available SiO2 in all depths were higher than the optimal range of soil properties for rice cultivation in Korea (NIAST, 2006). Also, SOM and CEC in all depths were within the optimal range, even though avail-P2O5 and exchangeable K were lower than that (Table 1).
Table 1.
Physicochemical properties of the point B soils at different soil depths.
‡Cation exchange capacity.
§Optimal range for rice cultivation (NIAST, 2006).
The study area was agricultural fields, indicating that crop productivity should be maintained, even after the introduction of soil remediation techniques. In the case of soil washing technique, one of the most prevalent soil remediation options practically applied in the field, chemicals used for this technique would destroy the basic nature and soil integrity, inducing detrimental effects on soil microbiology and fertility (Dickinson, 2000; Wu et al., 2010). Also, the application of the soil replacement technique with soil not suited to the original ecosystem resulted in the destruction of vegetation and habitat on agricultural land (Efroymson et al., 2004). In comparison with these techniques, it was expected that the soil environment for plant growth is little influenced by employing the soil reversal technique, due to both surface soil (0 - 50 cm) and subsoil (50 - 100 cm) mostly having similar physicochemical properties. As a result, we concluded that the Cd and Pb contaminated soil layers (0 - 50 cm) could be reversed with the deeper uncontaminated soil layers (50 - 100 cm).
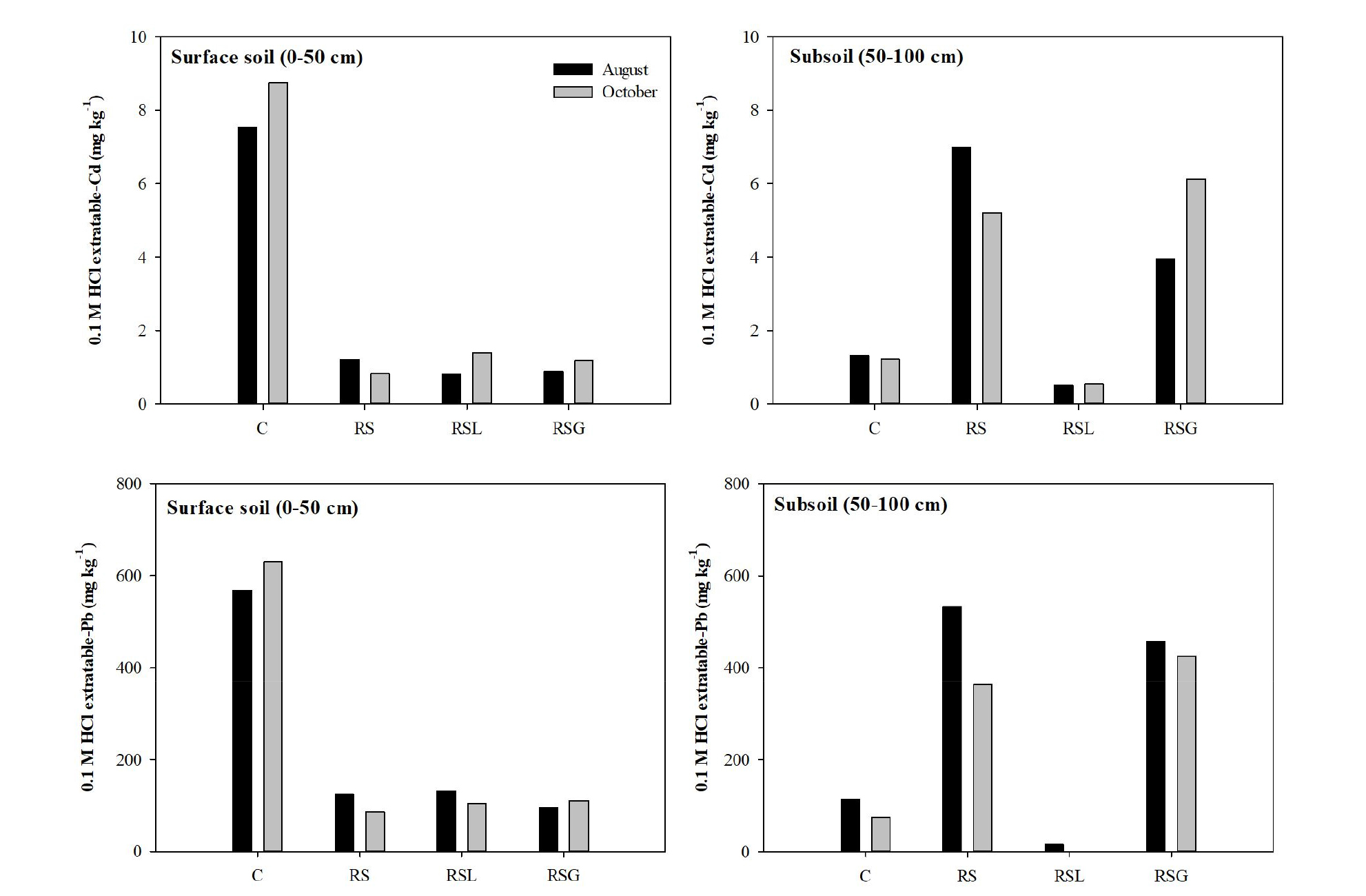
Changes in soil properties after the reversal process After the soil reversal treatment, the 0.1 M HCl extractable Cd and Pb concentrations in the surface soil and subsoil layers were successfully reversed. The 0.1 M HCl extractable Cd and Pb concentrations in the control (not reversed) surface soil (0 - 50 cm) and subsoil (50 - 100 cm) collected in August were 7.5 and 1.3 mg kg-1 for Cd, and 568 and 113 mg kg-1 for Pb. After the reversal, Cd and Pb concentrations of the reversed surface soil layer in RS were lower than that of control (Fig. 4). For example, 0.1 M HCl extractable Cd and Pb pools were 84 and 78 %, respectively, lower in the surface layer of RS in August, than those in the control soil; while the surface layer of RS had higher 0.1 M HCl extractable Cd and Pb concentrations than the control subsoil, as expected (Fig. 4).
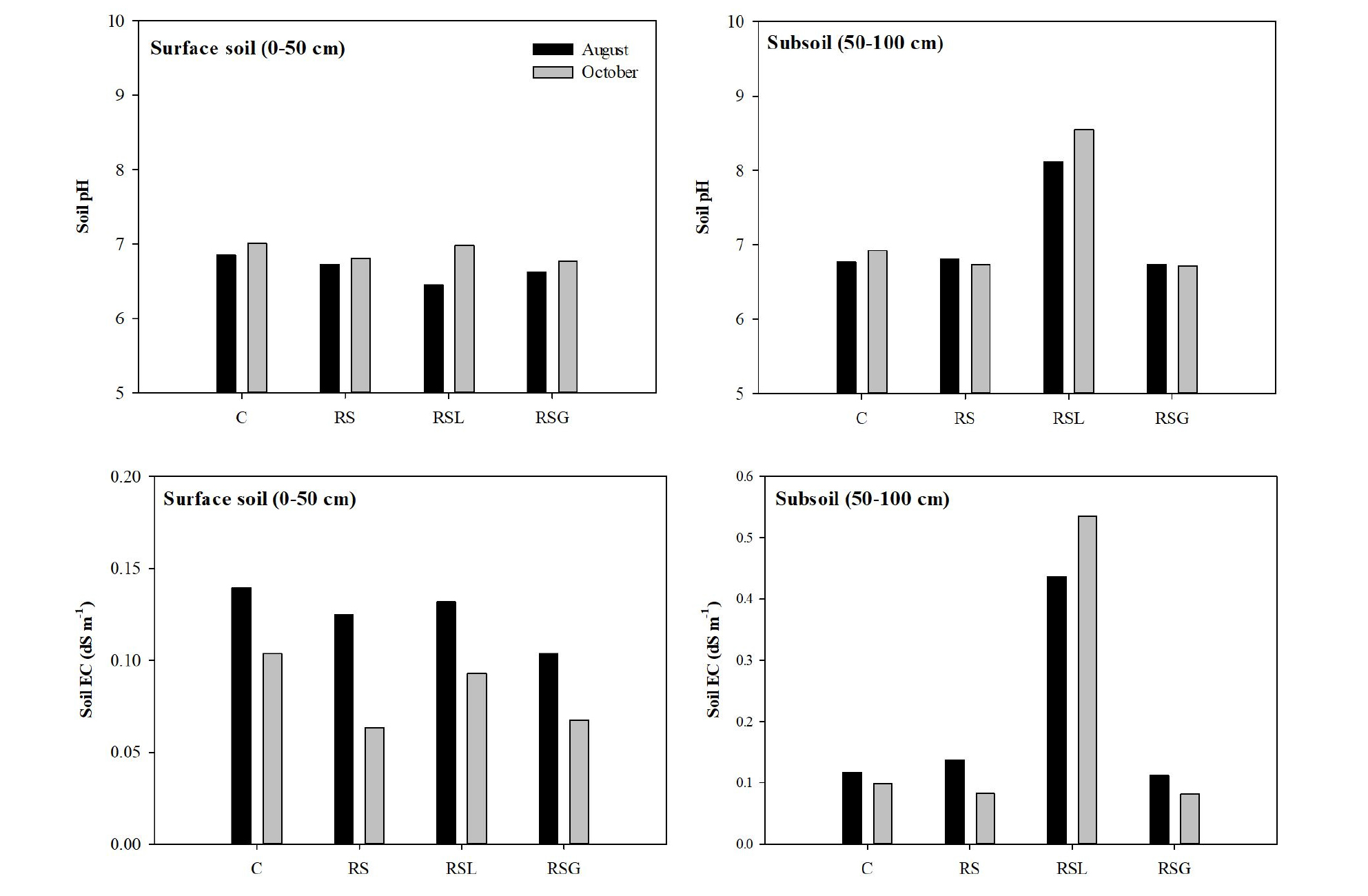
Also, in this study, the application of the soil reversal technique did not influence the surface soil pH and EC, when compared to the control soil (Fig. 5). The pH values of the RS collected in August and October from the surface were maintained in the range of 6.7 to 6.8, and these values were similar to those of the control surface soil collected at the same time (pH: 6.9 - 7.0). Like the soil pH, the EC values in RS surface soil samples collected in August and October (0.06 - 0.12 dS m-1) also had similar values to the control surface soil (0.10 - 0.14 dS m-1). Therefore, it was expected that the introduction of soil reversal has no or little influence on the chemical properties of the surface soil relating to plant productivity.
From these results showing that 0.1 M HCl extractable Cd and Pb concentrations were reversed between the RS and control soil, while both soils would show similar pH and EC, it was clearly confirmed that the surface layer and sub layer of the research area (point B) were completely reversed, following application of the soil reversal technique. Also, it was expected that compared to the control, rice cultivated in the RS showed high productivity due to the lower Cd and Pb concentrations in the RS surface layer, under similar soil properties for rice growth.
Generally, the capillary rise is defined as the upward flux leaving a static water table, due to crop transpiration and soil evaporation (Jorenush and Sepaskhah, 2003; Askri et al., 2010). For that reason, reversed surface soils may be affected by capillary rise of contaminated water from the reversed contaminated subsoils, resulting in recontamination of the surface soil. In this study, 0.1 M HCl extractable Cd and Pb concentrations of the studied surface soil were not different in August and October, indicating the Cd and Pb ions in the subsoil layer did not move up to the surface soil layer by the capillary rise. Therefore, the effect of both the lime layer and the gravel break layer addition could not be evaluated. However, the pH of the sub layer (50 - 100 cm) of RSL showed a different tendency to the RS and RSG (Fig. 5). In August, the sub layer of RSL had the highest pH value (pH: 8.1) among all the subsoil samples, while the pH of the RS and RSG was lower than that of control. In October, the pH of the RSL from the sub layer was 8.6, which was also the highest value, while the control soil pH was 6.9. This was due to the amount of lime (alkaline) that had moved down from the lime layer. Increases in soil pH can immobilize heavy metals in soil (Gray et al., 2006; Kim et al., 2015), because increased soil pH causes an increase of negatively charged surface where cationic metals can be absorbed (Naidu et al., 1994), and also leads to the precipitation of heavy metal ions as hydroxides or carbonates (Lee et al., 2009), which can decrease heavy metal bioavailability. Thus, in this study, increased subsoil pH resulting from lime addition induced increases in the immobilization of Cd and Pb. Consequently, as shown in Fig. 4, the 0.1 M HCl extractable Cd and Pb concentrations in the sub layer of RSL (0.6 mg kg-1 Cd and 1 mg kg-1 Pb) in October were lower than not only the RS (5.2 mg kg-1 Cd and 363 mg kg-1 Pb) and RSG (6.1 mg kg-1 Cd and 425 mg kg-1 Pb), but also the control soil (1.2 mg kg-1 Cd and 75 mg kg-1 Pb).
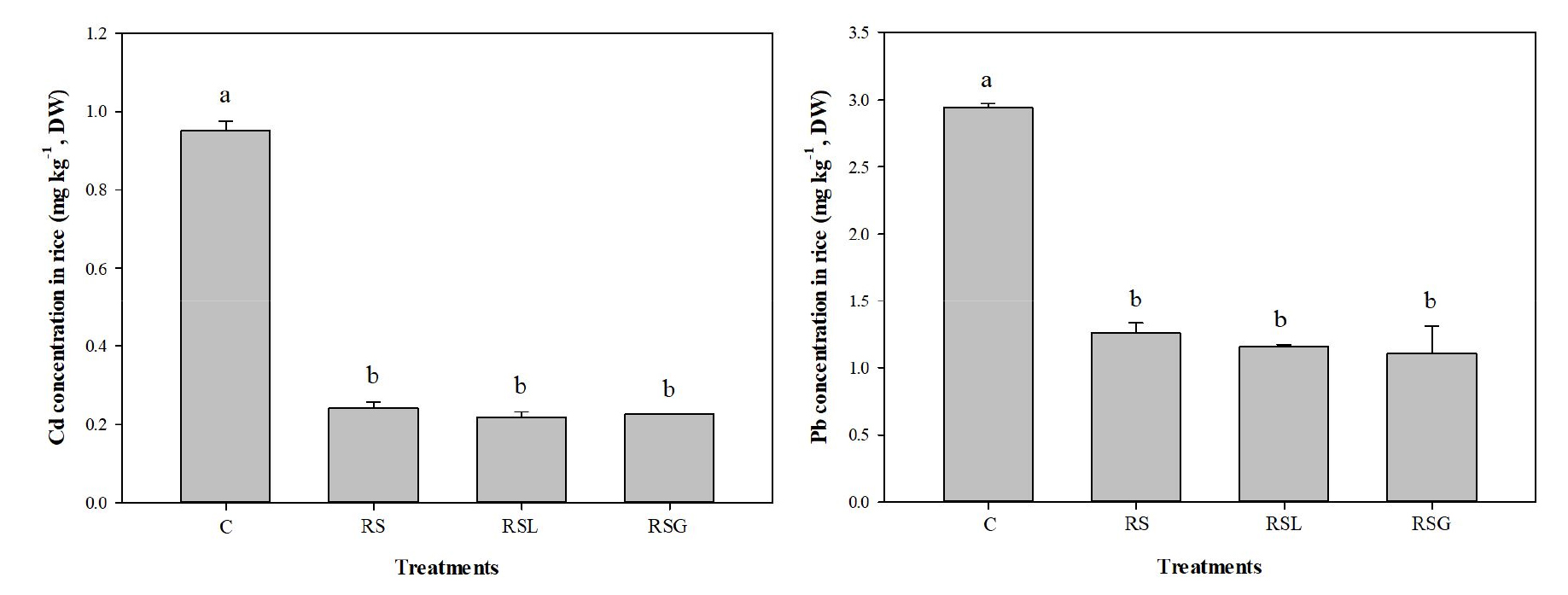
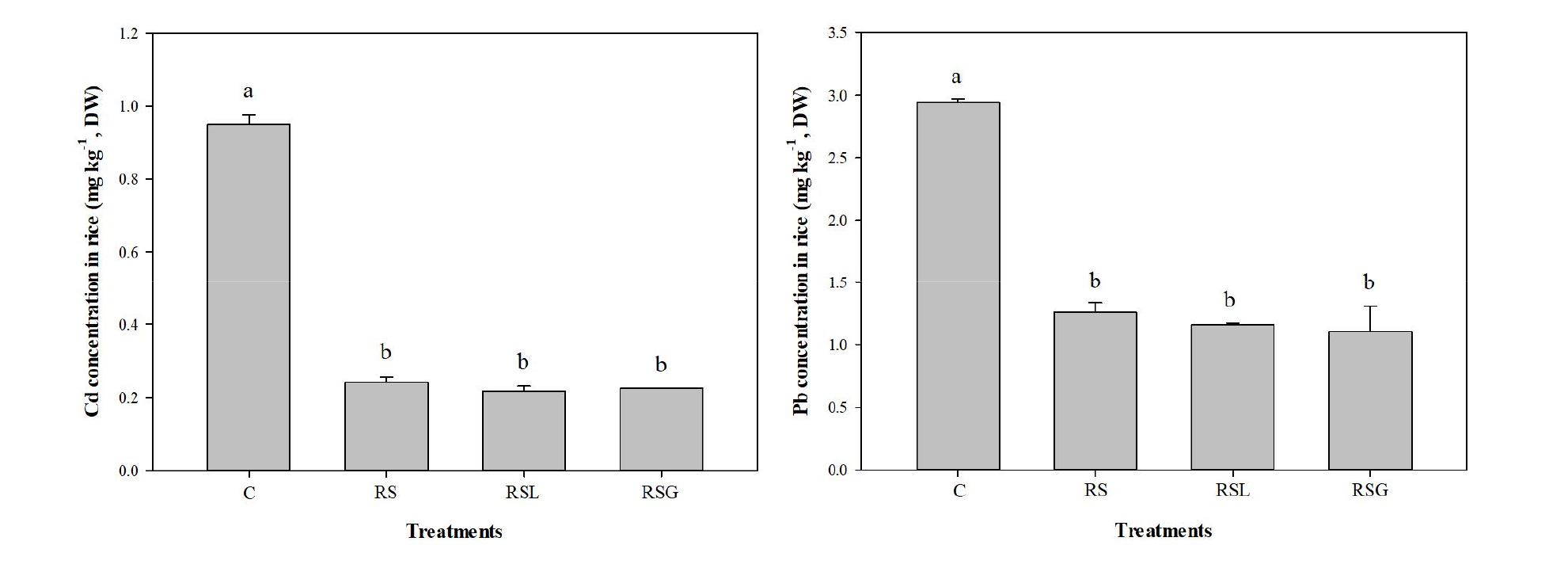
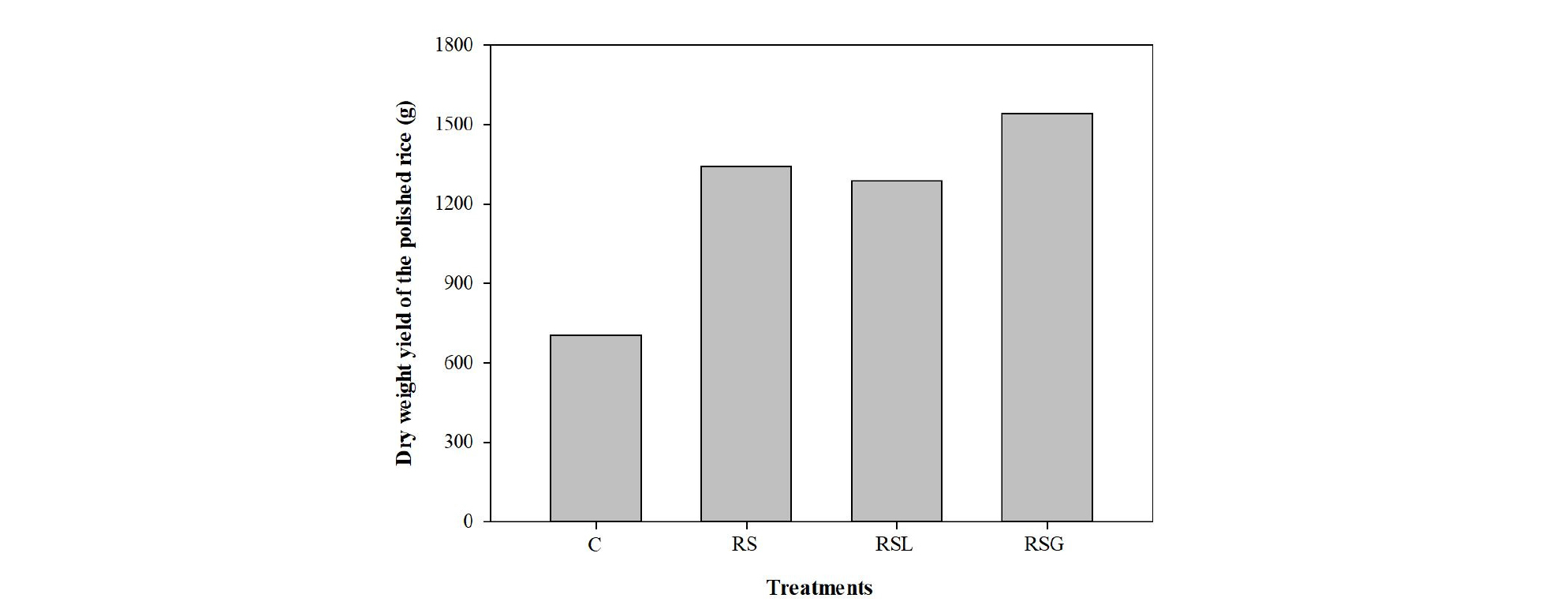
Cadmium and lead uptake by rice and its productivity As expected from the observed low in surface soil 0.1 M HCl extractable Cd and Pb pools following the soil reversal treatment, the average Cd and Pb concentrations in polished rice cultivated in the RS were lower than those in control (Fig. 6). For example, the accumulated Cd and Pb concentrations in polished rice cultivated in RS were respectively, 75 and 57 % lower than those of the control soil (0.95 mg kg-1 Cd and 2.9 mg kg-1 Pb). Cd and Pb concentrations in polished rice cultivated in the RSL and RSG were not significantly different from those in the RS. The rice productivity was also enhanced when the soil was reversed, due to the decreases in Cd and Pb uptake by rice. The total dry weight of polished rice cultivated in RS, RSL, and RSG were 1,342, 1,285, and 1,542 g which were 91, 83, and 119 %, larger yields of polished rice than those of the control soil (Fig. 7).
As mentioned in the Introduction, various soil remediation techniques have been developed, but these techniques are also known to have some limitations for agriculture. However, based on these proven results, the novel soil layer reversal technique can be used for remediation of agricultural paddy lands around abandoned mines, without problems associated with crop cultivation.
Conclusions
The current study demonstrated that soil reversal was a suitable candidate technique for heavy metal contaminated paddy soil near abandoned mines because this technique completely separated the less contaminated surface layer and the contaminated sub layer. Consequently, reduced both Cd and Pb concentrations in the rice examined, and increased amount of harvest. Specifically, RSL was a good option for this soil, because lime decreased 0.1 M HCl extractable Cd and Pb concentrations in subsoil by increasing the pH level of the subsoil, which meant the surface layer would be protected from recontamination by the rising of contaminated capillary water.